Background: Insufficient oxygen supply is associated with the pathophysiology of fetal growth restriction (FGR). Although the erythrocyte (RBC) is the most abundant and only cell type to deliver oxygen, its function and regulatory mechanism in FGR remains unknown. Recently, adenosine uptake by equilibrative nucleoside transporter 1 (ENT1), a key adenosine transporter expressed in RBCs, was reported to be crucial for RBCs to deliver oxygen. We aimed to investigate the involvement of RBCs' oxygen delivering capacity in maintaining fetal growth by focusing on RBC ENT1.
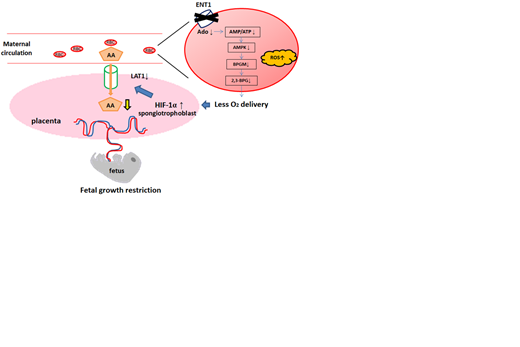
Methods and Results: The mating strategy was to delete ENT1 only on the maternal RBCs but not in the placentas or fetuses to assess the effect of maternal RBC ENT1 on fetal growth. Specifically, EpoR-Cre+ (EPO) female mouse was used as a control and Ent1f/f-EpoR-Cre+ (E1FE) female mouse as an experimental mouse and mated with WT male mouse. As a result, E1FE dams showed FGR phenotype with reduction of 12.9% in fetal weight compared to EPO group. The maternal RBCs showed decrease in p50 and 2,3-BPG in E1FE, indicating decreased oxygen delivery in E1FE RBCs.
To determine the molecular basis underlying the FGR phenotype seen in EIFE dams, we conducted a metabolomics screening of the RBCs isolated from controls and EIFE dams. It showed that adenosine metabolism inside the RBCs is the most impacted pathway. Specifically, it showed decrease in adenosine, AMP, and hypoxanthine, but adenine, ADP, and ATP did not show any reduction, implicating that ENT1-mediated uptake of adenosine is largely converted to AMP. We then incubated either WT or ENT1 KO RBCs with isotopically 13C15N labeled adenosine and traced the metabolism of intracellular adenosine derived from labeled adenosine. Indeed, adenosine was rapidly phosphorylated to AMP upon uptake, and 13C15N labeled AMP levels were lower in the ENT1 KO RBCs compared to controls. These findings provide evidence that 1) the most affected metabolic pathway in the RBCs of EIFE dam is adenosine metabolism; 2) ENT1-mediated uptake of extracellular adenosine is largely converted to AMP but not ATP.
We hypothesized that decreased AMP/ATP ratio underlies the reduced 2,3-BPG production by lowering AMPK activity and subsequently decreasing BPG mutase (BPGM) activity. We measured AMPK phosphorylation and BPGM activity in the RBCs from E1FE and EPO dams. Both AMPK and BPGM activity were decreased in RBCs of E1FE dams compared to controls. Thus, we conclude that i) adenosine derived from uptake via ENT1 is largely converted to AMP; ii) lack of maternal RBC ENT1 lowers AMP/ATP ratio and activity of AMPK and BPGM in maternal RBC.
We conducted immunofluorescence staining to assess hypoxia in the placentas, and confirmed the increased expression of HIF-1α in the placentas from E1FE dams. To determine functional changes of these placentas, we conducted metabolomics profiling in both the placenta and maternal plasma. Of all the metabolites altered, amino acids (AA) were the most reduced metabolites in E1FE placentas. In contrast, AA were the most accumulated in maternal plasma. We then injected isotopically labelled 13C15N AA mix in both controls and EIFE dams 24 hours prior to sacrifice. 13C15N AA level was decreased in the placentas in EIFE compared to the controls, while it was accumulated in plasma of EIFE, indicating reduced AA transporter function in the placentas of EIFE.
Finally, we performed real time PCR to quantify the mRNA of the known main transporters of AA in the mouse placenta. It showed reduction of LAT1 mRNA in E1FE placenta, where there was no difference in LAT2, SNAT1, or SNAT2. Western blot of the placenta lysates confirmed the expression of LAT1 was indeed reduced. To validate our mouse finding and determine if HIF-1α elevation directly induces LAT1 mRNA in humans, we treated cultured human trophoblast cell line (HTR-8/SVneo cells) with or without DMOG, a cell permeable prolyl-4-hydroxylase inhibitor. After confirming DMOG upregulated HIF-1α, we found stabilized HIF-1α induced LAT1 mRNA levels. Thus, we conclude that elevated HIF-1α underlies the reduction of LAT1 mRNA in cultured human trophoblasts.
Conclusion: Our findings suggest that maternal RBCs' oxygen delivering capacity mediated by ENT1 is essential for maintaining adequate placental oxygenation to support fetal growth via AA transporter function. Strategies to improve RBCs' function to deliver oxygen may provide new therapeutic possibilities for FGR.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal