Background: A common referral to hematology is for patients with suspected myelodysplastic syndrome (MDS) or cytopenias of undetermined origin. We recently implemented a Next Generation Sequencing (NGS) panel which covers a wide spectrum of genes related to the major myeloid disorders, including DNA based gene sequencing and RNA based gene fusion analysis, as a tier one clinical diagnostic test (Levy et al. Eur. J. Haematol 2019). We compared the diagnostic and prognostic information derived from conventional cytogenetics and NGS testing as well as the clinical impact on management in this patient group.
Methods: We identified all new cases with suspected MDS or cytopenias of undetermined origin referred between January 2018 to February 2019 that had both NGS and cytogenetic testing at London Health Sciences Centre, a tertiary care centre servicing a population of approximately 2.5 million in Southwestern Ontario, Canada. From the retrospective review of electronic medical record, patient demographics, diagnosis and management were ascertained. Diagnosis was based on the 2016 WHO classification and the ICD-10.Previously reported definitions for ICUS, IDUS, CHIP, and CCUSwere also adopted (Bejar et al. Leukemia 2017). Patients not meeting these criteria were defined as either cytopenia not yet determined (NYD) or secondary to other systemic disease. The impact of NGS and cytogenetics results on diagnosis, prognosis, and management of each disease were assessed by referring to the latest National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Either a bone marrow (BM) or peripheral blood (PB) sample from each patient was assessed by the Oncomine Myeloid NGS panel (Thermo-Fisher, MA, USA), which examines DNA sequence variants in 40 genes (17 full genes and 23 hotspot genes) along with an RNA-based panel of 29 fusion driver genes and their over 600 fusion partners. Patients' BM samples were also tested by the conventional cytogenetic G-banding method.
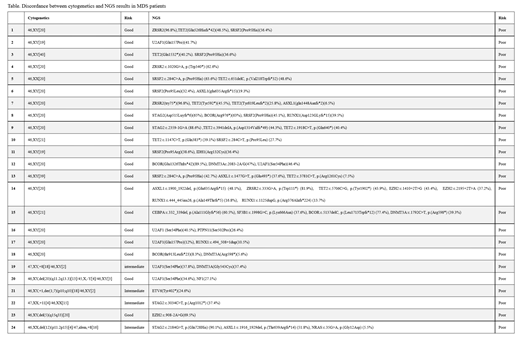
Results: Of the 1100 samples assessed by NGS, 178 met the study inclusion criteria. Overall, 120 (67.4%) patients had both cytogenetics and NGS performed on their BM samples. Of those 120 patients, 41 (34.2%) had DNA mutations, 17 (14.2%) had cytogenetic abnormalities, 22 (18.3%) had both molecular and cytogenetic findings, and 40 (33.3%) had neither abnormality identified. NGS Information contributed in diagnosing 13 (10.8%) patients, while cytogenetics in 6 (5%) patients. In addition, of 38 patients who were diagnosed as MDS with good/intermediate risk cytogenetics, 24 (63.2%) harbored poor prognostic risk mutations as detected by NGS (Table), influencing the management of disease. Additionally, 22 (12.4%) of all patients had NGS testing using PB and cytogenetics using BM samples, and of these 13 (52.2%) patients had NGS abnormalities. Finally, 36 (20.2%) of all patients had only NGS testing by PB samples. Of those, 12 (33.3%) were found to harbor at least one gene mutation including ASXL1, TP53, ZRSR2, and STAG1 suggesting poor prognostic significance for diagnoses of MDS, or JAK2, SF3B1 which could support a disease specific diagnosis.
Conclusion: NGS had enhanced diagnostic capabilities including classification of newly described entities such as ICUS or CCUS and more importantly yielded additional prognostic information compared to cytogenetics alone for this patient population. Cytogenetic findings were mainly aneuploidy or deletions, either clinically evident constitutional abnormalities such as Trisomy 21 and +X in Klinefelter syndrome, or loss of Y chromosome in a small proportion of cells and thus of questionable clinical significance. Based on this information consideration should be given to using the NGS panel as the primary molecular diagnostic and prognostic tool with karyotyping being reserved for subsets of patients being assessed for suspected MDS or cytopenias of undetermined origin.
Hsia:Amgen: Honoraria; Jansen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal