Background: Allogeneic hematopoietic cell transplantation (HCT) has been a successful strategy to treat myelodysplastic syndrome (MDS). With only approximately one-third of patients having an HLA matched sibling, most transplants use mismatched relative (haploidentical) or unrelated donors. In the current analysis we sought to study outcomes after haploidentical related compared to HLA-matched unrelated donor HCT for MDS (de novo or therapy-related).
Methods: We retrospectively studied 176 recipients of haploidentical related donor and 427 recipients of 8/8 HLA-matched unrelated donor HCT in the United States between 2012 and 2017. The primary outcome was overall survival. The effect of donor type on survival and other transplant outcomes were studied using a Cox regression model.
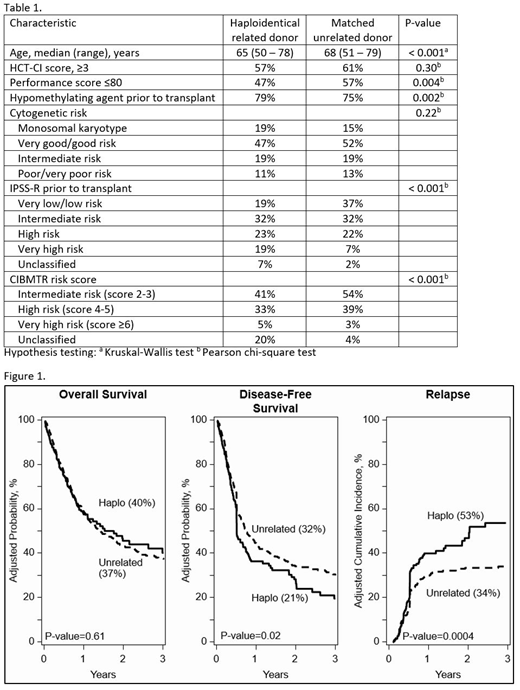
Results: Patient and disease characteristics are presented in Table 1. Most transplants (85%) were for de novo MDS in both donor groups. Although all patients received reduced intensity regimens, the predominant conditioning regimens were confounded by donor type. Total body irradiation (TBI) 200 cGy/cyclophosphamide/fludarabine (TBI/Cy/Flu; 82%) was the predominant regimen for haploidentical HCT and fludarabine with busulfan or melphalan (Flu/Bu or Flu/Mel; 79%) without in vivo T-cell depletion was the predominant regimen for unrelated donor HCT. Similarly, graft-versus-host disease (GVHD) prophylaxis was also confounded by donor type. Posttransplant cyclophosphamide/calcineurin inhibitor/mycophenolate (PT-Cy/CNI/MMF) was the prophylaxis regimen for all haploidentical transplants. CNI/MMF (31%) or CNI/methotrexate (69%) was used for unrelated donor transplants. Peripheral blood was the predominant graft for both donor types. The median follow-up was 24 months (range 3-77) after haploidentical and 36 months (range 3-74) after unrelated donor HCT. Results of multivariate analysis, adjusted for HCT-CI, prior treatment with hypomethylating agents (HMAs), and IPPS-R did not show differences in survival by donor type (HR 0.98, p=0.85; 40% vs. 37%), Figure 1. However, the relapse rate (adjusted for prior HMAs, IPSS-R, and recipient sex) was higher after haploidentical compared to unrelated donor HCT (HR 1.60, p=0.002, 53% vs. 34%), which led to lower disease-free survival after haploidentical HCT (HR 1.30, p=0.03; 21% vs. 32%), Figure 1. To further test the effect of regimen intensity, low dose TBI regimens were compared to Flu/Bu and Flu/Mel; we did not observe a difference in relapse risk (HR 0.95, p=0.76). Non-relapse mortality did not differ by donor type (HR 0.88, p=0.46). Interval between diagnosis and transplant was also not associated with outcomes. Acute grade II-IV acute GVHD (HR 0.46, p<0.001) and chronic GVHD (HR 0.34, p<0.001) was less common after haploidentical HCT. The 1-year graft failure rate was higher after haploidentical compared to unrelated donor HCT (15% and 8%, respectively, p=0.02).
Conclusion: Although the current analysis did not show differences in survival between haploidentical related and matched unrelated donor HCT, the higher relapse and consequently lower disease-free survival associated with the haploidentical HCT approach in this analysis (primarily TBI/Cy/Flu with PT-Cy/CNI/MMF) warrants caution. A more definitive comparison of the two donor types can be accomplished only if more haploidentical transplants were to use Flu/Bu or Flu/Mel conditioning.
Grunwald:Celgene: Consultancy; Pfizer: Consultancy; Agios: Consultancy; Merck: Consultancy; Abbvie: Consultancy; Medtronic: Equity Ownership; Incyte: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; Amgen: Consultancy; Trovagene: Consultancy; Cardinal Health: Consultancy; Janssen: Research Funding; Genentech/Roche: Research Funding; Novartis: Research Funding; Forma Therapeutics: Research Funding. Bolanos-Meade:Incyte Corporation: Other: DSMB fees. Bredeson:Otsuka: Research Funding. Gupta:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sierra Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Research Funding. Mussetti:Takeda: Honoraria; BMS: Honoraria; Novartis: Honoraria; Italfarmaco: Honoraria. Nakamura:Merck: Membership on an entity's Board of Directors or advisory committees; Celgene: Other: support for an academic seminar in a university in Japan; Alexion: Other: support to a lecture at a Japan Society of Transfusion/Cellular Therapy meeting ; Kirin Kyowa: Other: support for an academic seminar in a university in Japan. Nishihori:Novartis: Research Funding; Karyopharm: Research Funding. Solh:Celgene: Speakers Bureau; Amgen: Speakers Bureau; ADC Therapeutics: Research Funding. Weisdorf:Fate Therapeutics: Consultancy; Pharmacyclics: Consultancy; Incyte: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal