Introduction: Prior to effective novel agent (NA) approval for CLL, alloHSCT was recommended for CLL patients (pts) with early relapse/refractory disease after purine-analogs or deletion 17p (del17p) or TP53 mutation (TP53mut) based on expert consensus and retrospectively demonstrated overall survival (OS) advantage. Since 2014, approvals of ibrutinib (ibr), venetoclax (ven), and PI3K inhibitors (PI3Ki) have led to fewer alloHSCT for CLL. While NAs are indisputably effective, many pts will eventually progress through all available NAs. In the absence of data-driven consensus regarding role of alloHSCT for CLL, decision about proceeding to transplant is currently based on disease and transplant risk, response to NAs, and pt preference. This study of CLL pts who underwent alloHSCT following NA therapy (tx) aimed to help define the role of this potentially curative modality in the era of NAs.
Methods: This multicenter, retrospective cohort study examined CLL pts who underwent alloHSCT following treatment with ≥ 1 NA, including baseline clinical, prognostic, and transplant characteristics, tx preceding alloHSCT, transplant outcomes, and tx following alloHSCT. Complex karyotype (CK) and CLL status [complete remission (CR), partial remission (PR), stable disease (SD), and progression of disease (POD)] were defined per iwCLL criteria (Hallek, et al. Blood 2018). Univariate analyses utilizing COX regression evaluated association between pre-alloHSCT factors and progression free survival (PFS). PFS, OS, and non-relapse mortality (NRM) were estimated using Kaplan Meier and life table methods. Other statistics were descriptive.
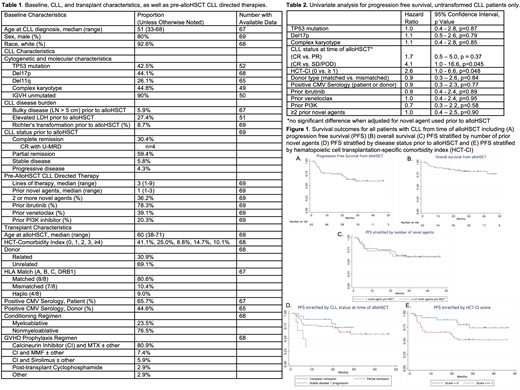
Results: 69 pts with CLL underwent alloHSCT following ≥ 1 NA across 14 US and EU centers, including 6 pts with Richter's transformation (RT) prior to alloHSCT. Table 1 describes baseline characteristics. Prior to alloHSCT, 78% received ibr (n=53), 39% ven (n=25), 20% PI3Ki (n=13), and 36% ≥ 2 NAs. 90% (n=62) received a NA immediately preceding alloHSCT [n=32 ibr (16% CR, 75% PR, 9% SD/POD), n=25 ven (52% CR, 40% PR, 8% SD/POD), 4 PI3Ki (75% PR, 25% SD/POD), 1 IMiD].
With a median (med) follow up 28 months (mo; range 1.2 -85), med PFS and OS from alloHSCT for the entire cohort were not reached (Figure 1A, B). PFS and OS for pts with CLL (excluding RT pts) from alloHSCT were 60% and 82% at 24 mo respectively. Poor risk disease characteristics (TP53mut, del17p, CK), prior NA exposure (ibr, ven, PI3Ki, ≥2 NAs), and transplant characteristics (matched (8/8) vs. mismatched (<8/8) donor, positive CMV serology) were not associated with inferior PFS (Table 2). NRM was 3.4% at D+100, 8.9% at 12 mo, 10.4% at 24 mo. Acute graft-vs-host disease (GHVD) was observed in 52% (med onset = D+49); moderate-severe chronic GVHD occurred in 26%. Fifteen deaths (22%) were observed due to POD (n=6), infection (n=7), GVHD (n=2). 21 pts relapsed following alloHSCT; post HSCT pts were treated with ibr (n=6), ven (n=6), rituximab (n=3), R-CHOP (n=2), R-HyperCVAD (n=1), second alloHSCT (n=1).
To guide decision making about timing of alloHSCT, we examined pts who received 1 (n=44) vs. ≥2 (n=25) NAs. These groups were similar in terms of poor risk features (del 17p 48% vs. 38%, TP53mut 44% vs. 40%, del11q 21% vs. 36%, CK 41% vs. 54%), transplant risk (med age 60 for both groups, med HSCT-CI 0 vs. 1, matched donor 86% vs. 71%), and disease status prior to alloHSCT (CR 25% vs. 40%, PR 66% vs. 48%, SD/POD 9% vs. 12%). PFS was similar for those exposed to 1 vs. ≥ 2 NAs (Figure 1C). Disease status at time of alloHSCT and hematopoietic cell transplantation-specific comorbidity index (HCT-CI) significantly impacted PFS (Figure 1D, E).
Conclusions: In the largest series of alloHSCT following NAs, data demonstrate that alloHSCT remains a viable curative strategy that can overcome adverse CLL characteristics including TP53 disruption and CK. As many pts treated with ibr and/or ven will progress or be intolerant, alloHSCT should be included in treatment algorithms for appropriate candidates. These data suggest that exposure to 1 vs. ≥2 prior NAs did not impact outcomes, though disease status at time of alloHSCT and HCT-CI are important predictors of PFS. Therefore, decision about proceeding to alloHSCT should consider comorbidities and current depth of response, as well as anticipated depth of response with the therapeutic options remaining. These data may significantly add to development of evidence-based guidelines for alloHSCT in the era of NAs.
Roeker:AbbVie: Equity Ownership; Abbott Laboratories: Equity Ownership. Brown:TG Therapeutics: Consultancy; Verastem: Consultancy, Research Funding; Sun Pharmaceuticals: Research Funding; Janssen: Honoraria; Teva: Honoraria; Morphosys: Other: Data safety monitoring board; Invectys: Other: Data safety monitoring board; Octapharma: Consultancy; Dynamo Therapeutics: Consultancy; Sunesis: Consultancy; Juno/Celgene: Consultancy; Genentech/Roche: Consultancy; Gilead: Consultancy, Research Funding; Catapult Therapeutics: Consultancy; AbbVie: Consultancy; Acerta Pharma: Consultancy; AstraZeneca: Consultancy; BeiGene: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Loxo: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Pharmacyclics: Consultancy. Dreger:AbbVie, AstraZeneca, Gilead, Janssen, Novartis, Riemser, Roche: Consultancy; AbbVie, Gilead, Novartis, Riemser, Roche: Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees, Other: Sponsoring of Symposia; Neovii, Riemser: Research Funding. Eyre:Roche: Honoraria; Abbvie: Honoraria, Other: Travel to Conferences; Takeda: Other: Travel to Conferences ; Gilead: Consultancy, Other: Research support, Speakers Bureau; Janssen: Honoraria, Other: Travel to Conferences . Brander:AbbVie: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Teva: Consultancy, Honoraria; Tolero: Research Funding; Acerta: Research Funding; TG Therapeutics: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Research Funding; Novartis: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy; BeiGene: Research Funding; DTRM Biopharma: Research Funding; MEI: Research Funding. Skarbnik:Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Verastem Oncology: Honoraria, Research Funding, Speakers Bureau; Kite Pharma: Honoraria, Speakers Bureau; Gilead Sciences: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Acerta: Research Funding; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Honoraria, Speakers Bureau; CLL Society: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Speakers Bureau; Novartis: Speakers Bureau. Coombs:H3 Biomedicine: Research Funding. Orchard:Pfizer: Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Unrestricted educational award for regional meetings ; Incyte: Other: Unrestricted educational award for regional meetings ; Adienne: Other: Unrestricted educational award for regional meetings . Sauter:Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy; Novartis: Consultancy; Celgene: Consultancy; Kite/Gilead: Consultancy; Precision Biosciences: Consultancy; Genmab: Consultancy; GSK: Consultancy. Giralt:Johnson & Johnson: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy; Kite: Consultancy; Amgen: Consultancy, Research Funding; Actinium: Consultancy, Research Funding; Novartis: Consultancy; Miltenyi: Research Funding; Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy. Perales:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria; Medigene: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Kyte/Gilead: Research Funding; Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Miltenyi: Research Funding; NexImmune: Membership on an entity's Board of Directors or advisory committees; MolMed: Membership on an entity's Board of Directors or advisory committees. Mato:Celgene: Consultancy; AbbVie: Consultancy, Research Funding; TG Therapeutics: Consultancy, Other: DSMB member , Research Funding; LOXO: Consultancy, Research Funding; DTRM Biopharma: Research Funding; Genentech: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Gilead: Research Funding; Acerta: Consultancy; Janssen: Consultancy; AstraZeneca: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Johnson & Johnson: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal