
With PTCy as GVHD prophylaxis, nonmyeloablative (NMA) HLA- haplo and HLA-matched blood or marrow (BMT) have comparable outcomes. Previous reports showed that discontinuation of immunosuppression (IST) as early as day 60 after infusion of bone marrow (BM) haplo allograft with PTCy is feasible. However, there are certain diseases in which PB may be favored over BM grafts to augment engraftment rates; however, given the higher rates of GVHD with PB, excessive GVHD becomes a concern with early discontinuation of IST. We present a completed, prospective single-center trial of stopping IST at days 90 and 60 after NMA haplo PB. (NCT02556931)
From 12/2015-7/2018, 117 evaluable patients (pts) with hematologic malignancies associated with higher rates of graft failure with PTCy (MDS, MPN, overlap syndromes, 2o AML, AML with MRD, MM, and CLL) received NMA PB allografts on trial. Haplo donors were preferred, but in patients lacking suitable haplo relatives, unrelated donors were employed with 6 in each IST cohort. The primary objective was to evaluate the safety and feasibility of reduced‐duration IST (from Day 5 through Day 90 in cohort 1 and through Day 60 in cohort 2.) Transplant inclusion criteria were standard and the conditioning included Cy (14.5 mg/kg IV D -6 and -5), fludarabine (D -6 to -2), TBI (200 cGy D -1) and T-cell replete PB. GVHD prophylaxis consisted of high-dose PTCy (50 mg/kg IV D 3 and 4), mycophenolate mofetil (D 5-35) and IST (tacrolimus/sirolimus) from D 5 forward. Priot to transplantation, pts were assigned to stop IST early if eligible, as defined by having ≥ 5% donor T cells at ~D 56 onward, no relapse, and no grade 2-4 acute or significant chronic GVHD. If ineligible to discontinue IST early, it continued through D 180. Monitoring rules declared reduced IST feasible if ≥ 33% of pts stopped IST early as planned. Safety stopping rules for early IST cessation were based on ≥ 5% graft failure, ≥ 5% NRM, ≥ 50% relapse, and ≥ 10% combined grade 3-4 acute GVHD and severe chronic GVHD, measured from the IST stop date to ~D 180. Historical data from 55 haplo transplants for MDS, CLL, and MPNs at our center using the same regimen and PB grafts informed safety calculations.
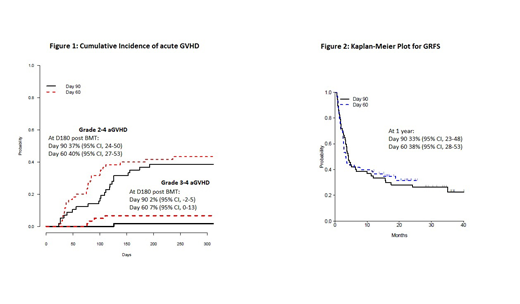
Of the 117 pts (median age 64 years, range 24-78), the most common diagnoses were MDS (33%), AML (with MRD or arising from antecedent disorder) (31%), MPNs (21%) myeloma (10%), and CLL (6%). By refined Disease Risk Index, 13% were low risk, 69% intermediate and 18% high. Shortened IST was feasible in 75 pts (64%) overall. Ineligibility for shortened IST was due most commonly to GVHD (17 pts), followed by early relapse (11 pts), NRM (7 pts), patient/ physician preference (4 pts) or graft failure (3 pts). Of the 57 patients in the D90 cohort (median follow up 35 mos), 33 (58%) stopped IST early as planned. Of the 60 patients in the D60 cohort (median follow up 20 mos), 42 (70%) stopped IST early as planned. The graft failure rate was 2.6%. NRM was very similar in the two arms, 12% at both 12 and 18 months in the D90 cohort and 10% and 13% at 12 and 18 months in the D60 cohort. Relapse in D90 cohort is 40% at 18 months compared to 33% at 18 months in the D60 cohort. Figure 1 shows cumulative incidence (CI) of acute grade 2-4 and grade 3-4 GVHD. Although the CI of grade 1-2 GVHD may be slightly higher in day 60 cohort, it is only 40% at D180. Severe chronic GVHD was 12% (D90) and 11% (D60) at 540 days. One year OS is 75% and 78% for the D90 and D60 cohorts, respectively. At 12 months PFS is 54% in the D90 group and 67% in the D60. At 12 months, the GRFS is 33% in the D90 group, and 38% in the D60 group. (Figure 2)
These data suggest that reduced-duration IST in pts receiving NMA haplo PB with PTCy is feasible and carries an acceptable safety profile. Risks of acute GVHD, chronic GVHD, graft failure and NRM appear similar to historical outcomes with IST until D180 and between the two cohorts. When comparing the D90 and D60 arms, grade 3-4, severe chronic GVHD, GRFS, OS and PFS were similar. Although a larger, prospective trial would be needed to uncover potential small differences in outcomes based on IST duration, these data show that similar to our findings with BM, many PB pts (64% in this trial) can discontinue IST as early as D60 without undue toxicity. The favorable toxicity profile of the PTCy platform, coupled with the feasibility and safety of early IST cessation, provides an ideal setting to incorporate novel post-transplantation approaches for relapse reduction.
DeZern:Astex Pharmaceuticals, Inc.: Consultancy; Celgene: Consultancy. Bolanos-Meade:Incyte Corporation: Other: DSMB fees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal