High dose melphalan (HDM) followed by autologous stem cell transplantation (ASCT) is standard of care after induction therapy for eligible patients with multiple myeloma (MM). However, ~40% of patients fail to achieve complete remission (CR), largely due to interpatient variability (~10-fold) in melphalan exposure (AUC). Thus, many patients experience subtherapeutic exposure and suboptimal response when using conventional body-surface-area (BSA)-based dosing. HDM, administered at a dose of 100mg/m2 on day -2 and -1, allows for potential modification of the day -1 dose if pharmacokinetic (PK) testing with rapid turnaround can be performed on day -2. Here we describe a clinically feasible, reproducible method of measuring melphalan PK that allows for real-time dose adjustments.
We developed and validated a robust melphalan assay with quality control for intra-day and inter-day precision, sample processing, and final LC-MS/MS analysis. MM patients aged ≥ 18 years undergoing HDM at a dose of 140 or 200 mg/m2 were eligible. HDM was administered days -2 and -1 prior to ASCT as a 30-minute infusion followed by saline flush. Blood for PK sampling (5ml aliquots), drawn at 10 specific time points post-infusion, were placed on ice and delivered to the PK lab for centrifugation and storage within 5 minutes of being drawn. PK parameters were determined from plasma concentration versus time data by a noncompartmental analysis using WinNonlin 8.1. Toxicities were assessed up to 30 days after ASCT using NCI-CTAE, version 5.0 grading. Patients were also followed for day 90 disease response rates.
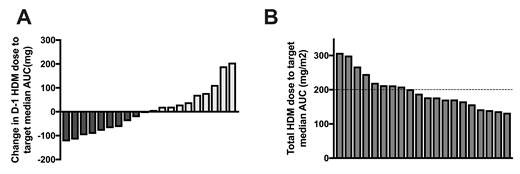
Twenty MM patients were enrolled with 18 receiving 200mg/m2 and 2 receiving 140mg/m2 due to end stage renal disease and age over 70 years. Using the first 5 patients, we confirmed that the AUC from day -2 correlated with day -1 (r=0.8), establishing that day -2 PK could be used to adjust the day -1 dose using a linear, dose-proportional calculation for future PK-directed studies. Notably there was considerable variation in day -2 AUC parameters, further confirming melphalan interpatient variability among patients. The median single-day AUC on day -2 was 7.49 (4.95-11.28) mg*h/L with an interquartile range of 2.66 mg*h/L. When comparing the upper and lower quartiles, there was a 1.85 fold higher single-day AUC (10.2 vs. 5.5 mg*h/L). Based on each patient's PK profile, we then calculated the theoretical dose for day -1 in order to target the total median melphalan AUC and compared it to the BSA-based dose received. The change in absolute melphalan dose on day -1 to achieve the total median AUC ranged from -124 mg to +206 mg (Figure 1A). When expressed as a BSA-based mg/m2 dose, the total 2-day dose would range from 133-308 mg/m2 (Figure 1B). Using D-optimality approaches we determined that reducing blood sampling from 8 to 4 time points did not affect AUC prediction, as confirmed by paired comparison tests comparing the mean AUCs between the new (4 point) sampling time schedule and the original (7.76 vs. 7.80 mg*h/L; p=0.8193).
Lastly, we examined clinical outcomes and whether AUC correlated with toxicity. One patient with a median single-day AUC of 10.58 mg*h/L died from sepsis. No patients developed grade 3/4 mucositis or GI-related toxicity. In modified Poisson regression, higher AUC correlated with increased risk of febrile neutropenia (RR 3.02, CI 1.00-9.26, p=0.05) and a trend towards increased use of antiemetics (RR 1.86, CI 0.95-3.65, p=0.07). Collection of disease response data is ongoing.
Here we describe the potential impact of performing rapid melphalan PK testing in a clinical setting. We report minimal intrapatient variability in melphalan exposure from day-2 to day-1 allowing a linear, dose-proportional method of dose-adjustment without need for a pre-transplant test dose or modification of the initial dosing regimen. Significant interpatient variability is observed, and dose adjustment simulations based on patients' PK data demonstrate that large, bi-directional dose adjustments are required to target a median AUC, emphasizing the importance of PK-directed HDM dosing. Our study posits that individualized melphalan dosing will both decrease interpatient variability and improve myeloma outcomes, and through allowing development of PK-directed HDM studies, suggests a novel approach to dosing in MM patients undergoing ASCT.
Galvin:Incyte: Consultancy. Quigley:Alexion: Membership on an entity's Board of Directors or advisory committees; Alnylam: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Dova Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; TEVA: Research Funding. Patel:Amgen: Consultancy, Speakers Bureau; Janssen: Speakers Bureau; Celgene: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal