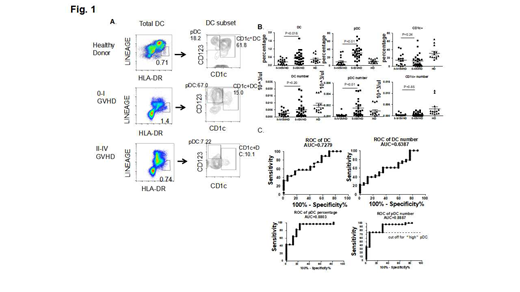
Graft-versus-host-disease (GVHD) remains a leading cause of transplant-related mortality (TRM) in patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT). Patients who do not respond to GVHD therapy are at high risk for death without relapse. Strategies that can predict the development of severe GVHD in transplant patients may improve risk stratification for steroids treatment refractory GVHD and TRM. Dendritic cells (DCs) play nonredundant roles in regulating both immunity and tolerance. Clinical and pre-clinical studies suggest that GVHD causes dysregulated development of functional DCs and impaired reconstitution of donor DCs is associated with increased risk of severe GVHD. We demonstrate that donor plasmacytoid DC (pDC) reconstitution around the engraftment time is a useful predictor for the development of severe GVHD in children after allo-HSCT. We examined reconstitution of donor DCs, including total DCs (lineage-negative (LIN-)HLADR+), pDCs (LIN-HLADR+CD123+CD1c-) and CD1c+DCs (LIN-HLADR+CD123-CD1c+), in peripheral blood of patients undergoing allo-HSCT, using age-matched healthy children as controls. The median time for engraftment was 14 days (range, 13.0~17 days), whereas clinical signs of GVHD occurred approximately 10 days later (median time, 24 days;range, 18~35 days). Thus, measuring the number and frequency of circulating blood DCs early at the engraftment time allowed us to evaluate the subsequent occurrence of GVHD. Transplant patients were followed up for 6 months after allo-HSCT. Of evaluable 45 transplant patients, 27 patients had no GVHD (grade 0) or mild GVHD (grade I), and 18 patients developed severe GVHD (grade II-IV GVHD). Patients who developed grade II-IV GVHD had no significant difference in frequency ( median, 0.2%; range, 0.04~0.35%) and number (median, 1.6 cell/ml; range, 1~3.8 cell/ml) of total DCs than patients with grade 0-I GVHD (median frequency, 0.41%; range, 0.15~0.63%, P<0.05; and median number, 2.6 cell/ml , range, 1~6.7 cell/ml,Figure1A,1B). Both pDCs and CD1c+DCs represent the major DC populations in human peripheral blood. Patients with grade II-IV GVHD showed significantly lower frequency and number of pDCs (median frequency, 3.4%; range, 0.64~14%, median number, 0.06/ml, range, 0.02~0.25 cell/ml) than patients with grade 0-I GVHD median frequency, 29.1%, range, 20.3%~39.2%, P<0.01; mediate number, 0.5 cell/ml, range, 0.2~1.9 cell /ml, P<0.01Figure1A,1B). In contrast, there was no significant difference in frequency and number of CD1c+DC between the two groups. These data suggest that the reconstitution of donor pDCs is linked to the occurrence of severe GVHD. Using Receiver operating characteristic (ROC) curve analysis, we explored thresholds of pDCs as a discriminator for grade II-IV GVHD patients versus grade 0-I GVHD patients. The median pDC level in all tested patients was 0.25 cell/ml (range, 0.1~0.9 cell/ml,Figure1C) at the engraftment time. We chose the threshold of pDC level at 0.3 cell/ml to evaluate the difference in patients with high (above the threshold) versus low (below the threshold) pDC levels. Of these 45 patients, 21 (46.7%) patients had high pDCs and 24 (53.3%) were low pDCs. Patients with low pDCs had a significantly higher probability of developing grade II-IV GVHD (p<0.01). The sensitivity of discriminating severe GVHD versus no/mild GVHD was 75% and the specificity was 94%. In addition, low engraftment pDC patients had higher TRM compared to high pDC patients (12.5% versus 0%) for median follow-up time 300 days (range, 210-465 days) after transplantation. However, there was no significant difference in overall survival rate between high pDC- and low pDC-group. Furthermore, the serum level of REG3α and ST2 at the engraftment time was not associated with the incidence of severe GVHD in these patients. Collectively, our findings identify that quantitation of pDCs at the engraftment time is a valuable prognostic predicator for severe GVHD in patients undergoing allo-HSCT. Given the importance of pDCs in modulating GVH reactions, it will be important to further determine whether impaired reconstitution of donor DCs may contribute to dysregulated immune tolerance against host tissues after allo-HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal