Introduction: Optimal HLA matching is associated with clinical outcome of unrelated donor (UD) hematopoietic cell transplantation (HCT)(Pidala, Blood2014, Morishima, Blood2015, Fürst, Blood2013), but a comprehensive analysis addressing this question in European transplant centers has not yet been performed. Within the CTIWP of EBMT, we have addressed this issue in adultsreceiving an UD-HCT from 2000 to 2015.
Methods: All consecutive cases of UC-HCT with available 6-loci high resolution (2nd field) HLA-A, -B, -C, -DRB1, -DQB1, -DPB1 typing for both patient and donor and ARD-level matching for at least 7/8 HLA-A,B,C,DRB1 alleles reported to the EBMT were selected. Further inclusion criteria were first allogeneic HCT for hematological malignancies, patient age >=18 years, availability of donor age and use of either bone marrow or peripheral blood (PB) as stem cell source. Overall, 9575 patient-donor pairs were included from 29 countries and 198 transplant centers.
Median follow-up was of 28.3 months, main diagnosis was acute leukemia (AL)(51.5%), disease stage was early in 44.1% of cases. UD-HCT were performed with PB in 84.7%, in vivo T cell depletion (TCD) in 64.4% and reduced intensity conditioning regimen in 57.3% of cases, and mostly standard graft-versus-host-disease (GvHD) prophylaxis with calcineurin inhibitors.
HLA data were validated using the HLAcore library and a haplotype based probability check from the German Donor Registry. Pairs were stratified by: 1) In the overall cohort, according to HLA-A, -B, -C, -DRB1 matching status (8/8 N=7724 and 7/8 N=1851) and 2) in informative 8/8 matched pairs (N=7480), according to HLA-DPB1 matching status as identical (23.7%), permissive (26.6%) or non-permissive (32.9%) by the 3-group T Cell Epitope (TCE3) model, or by the 4-group TCE4 model (Fleischhauer, Blood2017). Primary endpoint was overall survival (OS), secondary endpoints were non-relapse mortality (NRM), relapse and acute GvHD grade II-IV, and relapse free survival (RFS).
Results: At 5 years, OS and RFS in the entire cohort were 47% and 40.5%. The cumulative incidence of 5-y NRM, relapse and 1-y grade II-IV aGvHD was 28.1%, 31.4% and 19.4%, respectively.
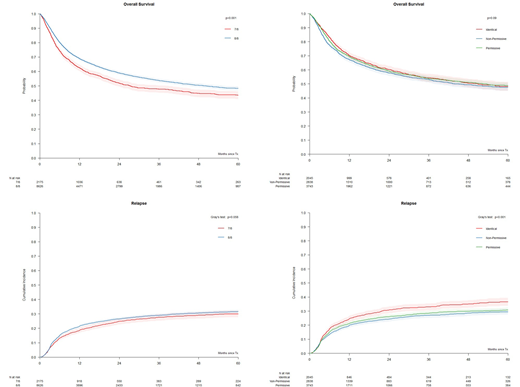
In multivariate analysis, a single mismatch at HLA-A, -B, -C, -DRB1 (7/8) was associated with a significantly higher risk of death compared to full match (8/8; HR 1.16, p<0.001). Other variables significantly associated with OS were patient (HR 1.14, p<0.001) and donor age (HR 1.08, p<0.001) per decade, CMV serostatus (HR 1.10, p=0.007), diagnosis of AL (HR 1.14, p<0.001), disease status (HR 1.22, p<0.001) and year of HCT (HR 0.98, p<0.001). The hazards of NRM, grade II-IV aGvHD and RFS were also significantly higher in 7/8 compared to 8/8 group (HR 1.34, p<0.001, HR 1.18, p<0.001 and HR 1.13, p<0.001, respectively) but not with lower risks of relapse (HR 0.96, p=0.51).
In 8/8 matched HCT, when comparing with the HLA-DPB1 TCE3 permissive group, NRM were significantly higher in the non-permissive but not in the allele matched group (HR 1.17, p<0.001 and HR 0.90, p=0.15). Permissive HLA-DPB1 mismatches were associated with significantly lower relapse risks compared to allele matches but not compared to non-permissive mismatches (HR 0.85, p<0.01 and HR 0.96, p=0.433, respectively). OS was not significantly different between permissively HLA-DPB1 mismatched and allele matched pairs (HR 0.98, p=0.678.) or non-permissively mismatched pairs (HR 1.07, p=0.08). RFS was similar between the 3 groups. Stratification according to the TCE4 group model resulted in similar outcome associations.
Conclusion: In this large independent cohort of UD-HCT from EBMT performed mostly from PB with in vivo TCD, a single allele mismatch at HLA-A, -B, -C, -DRB1 was independently associated with lower OS, and RFS, higher risk of NRM and aGvHD and no difference in relapse. The latter outcome was improved by permissive HLA-DPB1 mismatches in the 8/8 setting, which carried a significantly lower risk of NRM compared to non-permissive mismatches. The results from this new dataset validate current paradigms in donor selection and provide an important new platform for donor selection and HCT immunobiology.
Figure: OS and RI relapse in UD-HCT. Pairs were stratified according to A) 8/8 (N=7724) and 7/8 (N=1851) HLA-A,B,C,DRB1 allele mismatches, or B) HLA-DPB1 allele matches (N=2045), TCE3 permissive mismatches (N=3743) and TCE3 non-permissive mismatches (N=2838) in the 8/8.
Vago:Moderna Therapeutics: Research Funding; GenDx: Research Funding. Socie:Alexion: Consultancy. Kröger:Celgene: Honoraria, Research Funding; DKMS: Research Funding; JAZZ: Honoraria; Medac: Honoraria; Neovii: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Riemser: Research Funding; Sanofi-Aventis: Research Funding. Leleu:Takeda: Honoraria; Oncopeptide: Honoraria; Karyopharm: Honoraria; Amgen: Honoraria; Carsgen: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Janssen: Honoraria; BMS: Honoraria; Merck: Honoraria; Sanofi: Honoraria. Bonini:Novartis: Consultancy; -: Patents & Royalties: Adoptive T cell therapy field; Intellia Therapeutics: Research Funding; Molmed: Consultancy; Intellia Therapeutics: Consultancy; TxCell: Consultancy; GSK: Consultancy; Allogene: Consultancy; Kite/Gilead: Consultancy. Chabannon:EBMT: Other: Working Party Chair, Board member; Fresenius Kabi: Other: research support; Miltenyi Biotech: Other: research support; Terumo BCT: Other: speaker's fees; Celgene: Other: speaker's fees; Novartis: Other: speaker's fees; Gilead: Other: speaker's fees, hospitalities; Sanofi SA: Other: research support, speaker's fees, hospitalities.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal