Background
Fecal microbiota composition is associated with important outcomes after allo-HCT including survival, relapse, GVHD, and infections. We previously demonstrated in a multicenter observational study that HCT patients present with fecal microbiota configurations that have lower diversity and are distinct from those of healthy individuals, and that pre-HCT microbiota injury predicts poor overall survival. Here, we hypothesized that pre-HCT fecal microbiota features predict development of critical illness post-HCT.
Methods
We analyzed 828 adults who received a first allo-HCT from 2009 to 2017 at a single institution who had an evaluable fecal sample in our biobank collected within the 10 days prior to cell infusion. The patients were heterogeneous with respect to transplant indication, conditioning intensity, graft source (cord blood, peripheral blood, marrow) and graft manipulation (CD34-selection). The V4-V5 regions of 16S rRNA genes of DNA extracted from fecal samples were amplified and annotated taxonomically. The outcome of interest was time to ICU admission, which was assessed using survival-analysis methods. The reason for admission to the ICU was evaluated for each subject.
Results
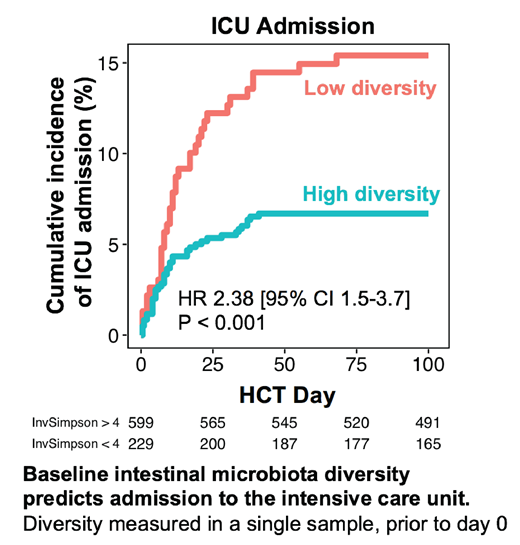
Seventy-five (9%) patients were admitted to the intensive care unit (ICU) between the day of cell infusion and day +50; the peak incidence of ICU admission occurred on day +10. The most common indications for ICU admission were respiratory failure (65%) and infection (27%). Patients were stratified based on fecal microbiota diversity, as assessed by 16S sequencing of stool samples collected prior to transplantation, into high (inverse Simpson index ≥4) and low (<4) diversity groups following a previously-published cutoff. Patients with low diversity pre-HCT had a strikingly higher risk of ICU admission than those with high diversity (HR 2.38 [95% CI 1.5-3.7], p <0.001, see the Figure). This association remained significant in a multivariate Cox proportional hazard model that accounted for conditioning intensity, graft source, graft manipulation, and the HCT-CI comorbidity index (multivariate p = 0.003). HCT-CI score was also an independent predictor of ICU admission. The association between pre-HCT fecal diversity and ICU admission was also significant when the outcome definition was limited to ICU transfers for reason of respiratory failure or sepsis (to the exclusion of such indications as hemorrhage, anaphylaxis, or isolated dysfunctions of the cardiac, renal, or neurological systems).
Conclusion
Pre-transplant fecal microbial diversity is an independent predictor of intensive-care-requiring critical illness in the post-HCT period. These observations highlight the pre-HCT period as a window of opportunity to (a) assess microbiota injury in conjunction with comorbidity evaluation, (b) inform selection of antibiotic prophylaxis, gut-decontamination, GVHD-prophylaxis, or conditioning regimens, and (c) intervene with microbiota injury-remediation or prevention strategies.
Brereton:Seres Therapeutics: Other: Salary Support. Clurman:Seres Therapeutics: Research Funding. Slingerland:Seres Therapeutics: Other: Salary supported by Seres funding. Shah:Janssen Pharmaceutica: Research Funding; Amgen: Research Funding. Scordo:McKinsey & Company: Consultancy; Angiocrine Bioscience, Inc.: Consultancy. Politikos:Angiocrine Bioscience Inc: Research Funding. Gyurkocza:Actinium Pharmaceuticals: Research Funding. Barker:Angiocrine Bioscience Inc: Research Funding; Gamida Cell: Research Funding; Merck: Research Funding. Perales:Kyte/Gilead: Research Funding; Miltenyi: Research Funding; MolMed: Membership on an entity's Board of Directors or advisory committees; NexImmune: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria; Medigene: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees. Giralt:Amgen: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy; Actinium: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Kite: Consultancy; Johnson & Johnson: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy; Miltenyi: Research Funding; Takeda: Consultancy, Research Funding; Novartis: Consultancy. van den Brink:Merck & Co, Inc.: Consultancy, Honoraria; Acute Leukemia Forum (ALF): Consultancy, Honoraria; Magenta and DKMS Medical Council: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Other: Licensing; Amgen: Consultancy, Honoraria; Therakos: Consultancy, Honoraria; Seres Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Flagship Ventures: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Evelo: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria. Pamer:Bristol Myers Squibb: Honoraria; Celgene: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; MedImmune: Honoraria; Novartis: Honoraria; Ferring Pharmaceuticals: Honoraria. Peled:Seres Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal