Natural killer (NK) cells are an emerging cellular immunotherapy for patients with acute myeloid leukemia (AML); however, the best approach to maximize NK cell anti-leukemia potential is unclear. Paradigm-shifting reports have shown that NK cells exhibit "memory-like" properties following hapten exposure, virus infection, or combined cytokine pre-activation. Human cytokine-induced memory-like (ML) NK cells display enhanced re-stimulation responses to numerous activating stimuli, including tumor target cells. This has been translated in clinical trials as cellular therapy for rel/ref AML patients (NCT#), and the dose escalation of a phase 1/2 study has been completed (PMID). Donor memory-like NK cells expanded in patients' blood and bone marrow and retained enhanced functionality ex vivo, with 7 of 11 patients achieving CR/CRi. Since NK cell recognition depend on signals from multiple activating and inhibitory receptors, we developed mass cytometry panels to immunophenotype and track the diversity and effector functions of these human in vivo-differentiated memory-like NK cells. Previous work showed that that in vivo-differentiated memory-like (ML) NK cells were distinct from baseline (BL) NK cells from the same donor, as well as NK cells from normal donor PBMC. Multidimensional analyses revealed a memory-like phenotype: CD56hi CD11blo CD62L+ NKG2Ahi NKp30hi Ki-67+. Furthermore, Citrus analyses revealed that higher NKG2A expression was significantly correlated with treatment failure. NKG2A is a C-type lectin receptor with two immunoreceptor tyrosine-based inhibitory motifs. Signaling through NKG2A is achieved when it engages its ligand, HLA-E. HLA-E is a non-classical major histocompatibility complex class I molecule that is expressed abundantly on many normal tissue types as well as tumors, including AML. Based on these findings that NKG2A is upregulated on memory-like NK cells and the intensity of NKG2A on memory-like NK cells correlated with patient responses, we hypothesized that NKG2A/HLA-E interactions represent a major barrier to memory-like NK cell responses.
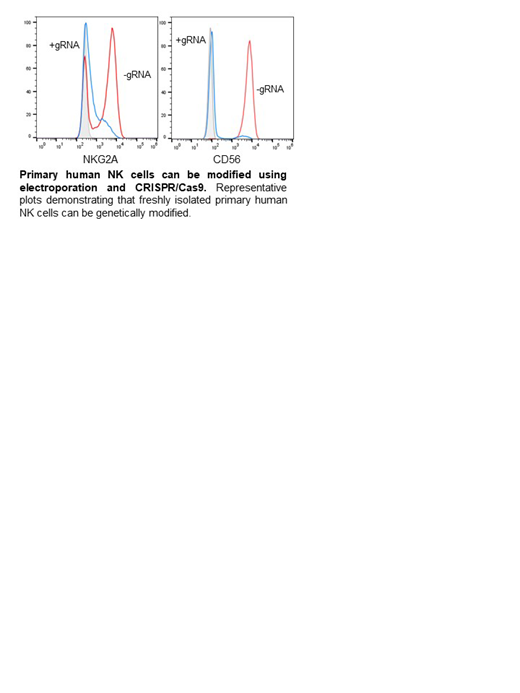
CRISPR based gene editing of primary human NK cells has been technically challenging. In order to interrogate the role NKG2A may play in limiting ML NK cell responses, we optimized the MaxCyte GT electroporation system to introduce Cas9 and guide RNA into freshely isolated, purified human NK cells. As proof of principle, we introduced Cas9 and gRNA targeting CD56 into NK cells and assessed CD56 expression a week later. We observed a 96.5% ± 0.8% (SD) reduction in median CD56 expression as determined by flow cytometry, with little impact on cell viability (90.3% ± 2.7% live v 87.0% ± 3.1% live ΔCD56) after electroporation. Next we introduced Cas9 and gRNA against NKG2A into freshly isolated, purified human NK cells. After electroporation, cells were briefly incubated with IL-12/IL-15/IL-18, overnight. The cytokines are washed away and the cells incubated for 4 days in low-dose IL-15, which was required for their survival. NKG2A frequency was decreased 64.72% (ΔNKG2A v Control; 42.3-85.7% range, ± 13.18% SD) by 4 days post-electroporation. We compared the ability of these cells to respond to HLA-E+ K562 leukemia targets and observed a significantly enhanced ML NK cell response by ΔNKG2A ML NK cells compared to control ML NK cells (19.04 ± 5.9% IFN-γ+ v 34.9 ± 8.8% ΔNKG2A IFNγ+; Mean ± S.D.). Finally, we infused ΔNKG2A ML NK or control ML NK cells into NSG recipient mice and assessed the spleen at D7 and D14 for persistence and NKG2A expression. We were able to detect ΔNKG2A ML NK and control ML NK cells at both time points and the ΔNKG2A ML NK cells remain NKG2A-negative, post-transfer. Using gene-editing approaches, the data reveal an important inhibitory role for NKG2A on ML NK cell responses against HLA-E+ targets.
Primary human NK cells are notoriously difficult to modify by virus or electroporation. Indeed, most reports utilize expanded NK cells or cord-blood differentiated NK cells which were edited in the stem cell stage. This report demonstrates that mature NK cells can be modified with little ex vivo manipulation with high efficiency and viability. This method has broad potential to expand our understanding of human NK cell biology using genetic loss or gain of function techniques, as exemplified by identification of NKG2A as a critical ML NK cell checkpoint.
Cooper:Wugen: Consultancy, Equity Ownership, Patents & Royalties. Fehniger:Cyto-Sen Therapeutics: Consultancy; Horizon Pharma PLC: Other: Consultancy (Spouse).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal