
Purpose: The roles of allogeneic vs autologous SCT for pts with relapsed FL are under debate. Past studies suggested that allogeneic SCT has a lower relapse rate due to a graft-versus-lymphoma effect, but the risks of graft-versus-host disease (GVHD) and early death negated any survival benefit over ASCT. NMAT allows the performance of transplants with a lower toxicity. Efforts to answer address the debate were attempted through a prospective Blood and Marrow Transplant Clinical Trials Network randomized trial; however it was closed early due to a low accrual. In this report, we compared the outcomes of NMAT vs. ASCT in pts with relapsed FL at a single center.
Methods: Pts with relapsed FL received an NMAT if they had an 8/8 HLA-matched adult donor. Pts who had no suitable donors or were not able to have an NMAT due to Medicare/Medicaid reimbursement guidelines received instead an ASCT if they had chemo-sensitive disease (PR or CR) and no bone marrow involvement by disease. Organ eligibility criteria were comparable between the two transplant groups. Conditioning for NMAT consisted of fludarabine (30 mg/m2 daily x3), cyclophosphamide (750 mg/m2 daily x3) (or bendamustine 130 mg/m2 daily x3), rituximab (375 m2 day -13, then 1000 mg/m2 days -6,+1 and +8) +/- Yttrium-90 ibritumomab tiuxetan (0.4 mCi/kg) (Blood 2012 ;119:6373; Blood 2014;124:2306). Tacrolimus and mini-methotrexate were used for GVHD prophylaxis. In addition, thymoglobulin of 1 mg/kg was given on days -2 and -1 in patients receiving a matched-unrelated donor (MUD) transplant. Pts with ASCT underwent chemo-mobilization of stem cells with rituximab for in-vivo purging; rituximab was also given on days+1 and +8 with BEAM conditioning (Clin Cancer Res 2018; 24:2304-11). This study was IRB-approved at our center.
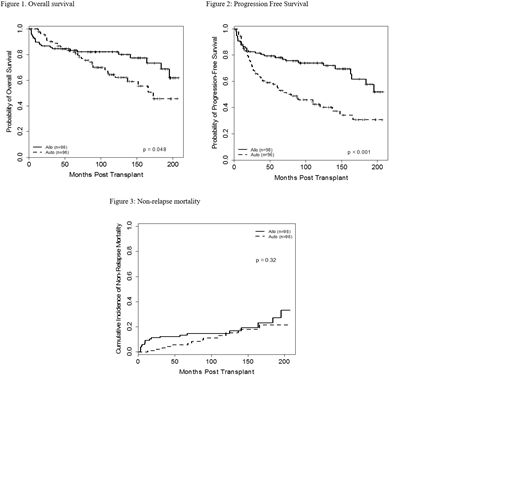
Results: The study included 98 NMAT and 96 ASCT pts treated between 2000-2017. Median age was 53 and 56 yrs, respectively and 24 (24%) and 32 (33%) pts were older than 60 yrs (P=0.21). Male gender was 50% vs 48%, respectively. Eleven NMAT pts (11%) and 18 ASCT (19%) pts had an HCT-CI of > 4 (P=0.16). The time from diagnosis to transplant was 38 months in both groups. Rituximab-chemo induction was received in 54 (55%), and 66 (69%) pts at diagnosis, respectively (P=0.056). Seventy-one NMAT (72%) and 61 ASCT (64%) pts relapsed within 2 yrs of their induction chemotherapy (P=0.22). Most pts (94% and 100%) received transplant from peripheral blood. Significant differences between treatment groups were observed for disease status, prior number of chemotherapies, and year of SCT. More pts receiving NMAT had refractory disease compared with the ASCT group (P=0.018). In addition, a higher percentage of NMAT pts had bulky disease (P=0.016), had a transplant > 1st relapse (P=0.001), received > 3 prior chemotherapies (P=0.003), and had a transplant between 2000-2005 rather than later years (P=0.033) compared with the ASCT pts. NMAT transplant characteristics included MUDs in 28 (29%) patients, 42 (43%) ABO-mismatched and CMV was reactive in 80% of pts and/or donors. With a median follow-up for NMAT pts of 98 months (range, 3-208 months) and 94 months (range, 1-207 months) for ASCT pts, overall survival was significantly better for NMAT patients compared to ASCT patients (62% vs 46%; P=0.048) (Figure 1). Similarly, progression-free-survival was better for NMAT pts compared to ASCT pts (52% vs 31%; P <0.001) (Figure 2). The cumulative incidence (CI) of relapse was 15% vs 48%, respectively (P<0.0001).The significant differences were also seen in propensity score matched analysis where the two groups were matched on age, bulk, disease status, number of prior therapies, induction chemotherapy and year of transplant. The OS and PFS benefits of NMAT were also observed for pts >60 yrs of age. The CI of grade II-IV and III-IV acute GVHD in the NMAT group was 22% and 9%, respectively. The CI of chronic extensive GVHD was 38%. The 8-year CI of secondary MDS/AML in the ASCT group was 12%, which progressively increased to 21% at end of assessment. Non-relapse mortality was similar between the two groups (Figure 3).
Conclusions: This is the first study to show that NMAT confers a superior survival in pts with relapsed/FL compared with ASCT. Our conclusions are supported by long-term follow-up. A collaborative effort is needed to allow Medicare/Medicaid pts with high-risk relapsed FL to benefit from NMAT.
Nastoupil:Spectrum: Honoraria; TG Therapeutics: Honoraria, Research Funding; Novartis: Honoraria; Janssen: Honoraria, Research Funding; Gilead: Honoraria; Bayer: Honoraria; Celgene: Honoraria, Research Funding; Genentech, Inc.: Honoraria, Research Funding. Jabbour:Pfizer: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Amgen: Consultancy, Research Funding. Bashir:Amgen: Membership on an entity's Board of Directors or advisory committees; Imbrium: Membership on an entity's Board of Directors or advisory committees; Spectrum: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; StemLine: Research Funding; Acrotech: Research Funding; Celgene: Research Funding. Ciurea:Miltenyi: Research Funding; Spectrum: Membership on an entity's Board of Directors or advisory committees; MolMed: Membership on an entity's Board of Directors or advisory committees; Kiadis Pharma: Membership on an entity's Board of Directors or advisory committees, Other: stock holder. Molldrem:M. D. Anderson & Astellas Pharma: Other: Royalties. Oran:Astex pharmaceuticals: Research Funding; AROG pharmaceuticals: Research Funding. Popat:Bayer: Research Funding; Incyte: Research Funding; Jazz: Consultancy. Qazilbash:Bioclinica: Consultancy; Amgen: Other: Advisory Board; Autolous: Consultancy; Speaker: Other: Speaker.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal