Donor T cells present in the stem cell or bone marrow inoculum are critical to the induction of acute graft-versus-host disease (aGvHD). AGvHD of the gastrointestinal (GI) tract that is non-responsive to corticosteroid therapy is significant problem for patients undergoing allogeneic hematopoietic stem cell (allo-HSCT) transplantation with less than 15% of these patients alive one year after diagnosis. Previously, we have shown that the infusion of donor innate lymphoid type II (ILC2) cells could prevent and treat acute GvHD of the lower GI tract. This approach for clinical translation is cumbersome as it would require the generation of donor derived ILC2 cells for each recipient. Thus, the ability to use third party ILC2 cells would provide an "off the shelf" reagent that could be used to prevent aGvHD.
Here, we show that third party ILC2 cells can effectively prevent and treat lower GI tract aGvHD. Co-transplantation of a single dose of third party ILC2s with MHC completely mismatched donor T cells significantly improved median survival of recipients by 11 days and reduced the clinical scores in mice. However we did not observe an improvement in overall survival (OS). When we evaluated a mechanism for this, we found that third party ILC2 cells did not persist in GvHD target organs.
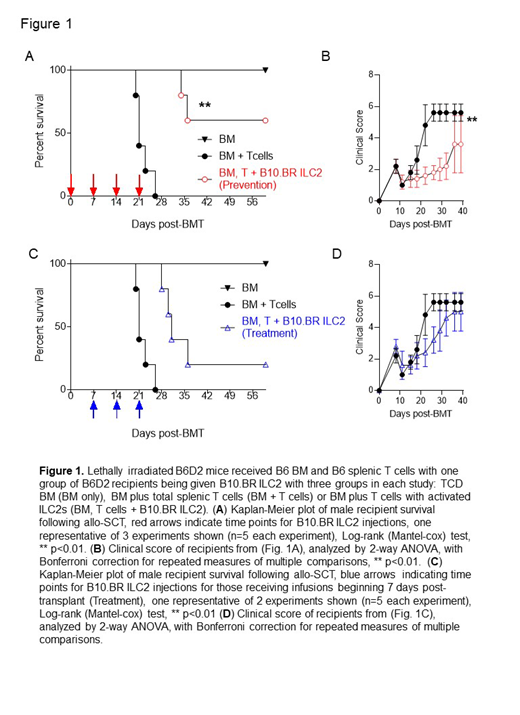
To overcome issues with persistence, we infused multiple doses of third party ILC2s on days 0, 7, 14, and 21 in recipient mice receiving complete MHC mismatched donor T cells and BM. Multiple infusions during early aGvHD significantly increased survival rates (Fig. 1A) and significantly reduced clinical scores (Fig. 1B) of recipients post-BMT, with 60% of the recipients surviving more than 60 days.
Prevention of aGvHD with ILC2s can be clinically beneficial. However, it would be more advantageous if third party ILC2s could be used to treat active aGvHD. To evaluate their therapeutic benefit, ILC2s were injected 7 days after transplant, a time when donor T cells are activated in GvHD target organs, with additional injections at days 14 and 21. Interestingly, infusion of ILC2s as a treatment increased overall survival, increasing median survival by 10 days and reducing clinical scores from day 20 to day 30 compared to untreated recipients (Fig. 1C and 1D). These findings demonstrate multiple infusion of third party ILC2 cells can prevent and treat acute GvHD.
To evaluate the mechanism of activity of ILC2 cells, we utilized donor BM cells lacking expression of the IL-13 receptor. Prevention of aGvHD was significantly diminished with the donor BM cells lacked the ability to respond to IL-13. Additionally, we found using FoxP3-DTR mice that the absence of Tregs modestly but significantly diminished the function of ILC2 cells. Thus, our data are consistent with IL-13 generation by ILC2 cells acting on donor myeloid-derived suppressor cells and Tregs, which greatly extends our initial findings. Finally, we show that ILC2 cells in vitro are not killed by corticosteroids paving the way for clinical trials for steroid non-responsive patients.
Stefanski:Novartis: Consultancy, Speakers Bureau. Blazar:RXi Pharmaceuticals: Research Funding; Alpine Immune Sciences, Inc.: Research Funding; Fate Therapeutics, Inc.: Research Funding; Magenta Therapeutics and BlueRock Therapeuetics: Membership on an entity's Board of Directors or advisory committees; Regeneron Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Kamon Pharmaceuticals, Inc: Membership on an entity's Board of Directors or advisory committees; Five Prime Therapeutics Inc: Co-Founder, Membership on an entity's Board of Directors or advisory committees; Tmunity: Other: Co-Founder; BlueRock Therapeutics: Membership on an entity's Board of Directors or advisory committees; Abbvie Inc: Research Funding; Leukemia and Lymphoma Society: Research Funding; Childrens' Cancer Research Fund: Research Funding; KidsFirst Fund: Research Funding. Serody:Merck: Research Funding; GlaxoSmithKline: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal