A potential role for endogenous innate lymphoid cells (ILCs) in prevention of gastrointestinal (GI) GVHD has recently been described in murine and clinical models, and experimental approaches for adoptive cellular therapy with ILCs have recently been described as well. However, the specific in vivo roles of tissue-resident ILCs and their capacity for reconstitution post-transplant remain unclear. We thus sought to characterize the significance and tissue distribution of ILCs after allogeneic bone marrow transplant (allo-BMT) in mouse models and in patients.
In order to specifically assess the functional role of ILCs after allo-BMT, we established a clinically relevant acute GVHD model based on sex mismatched H-Y antigens. Lethally irradiated Rag2-/-male mice (Thy1.2, B6) were transplanted with T cell-depleted (TCD) BM and purified T cells obtained from wild-type female mice (Thy1.1, B6). To specifically deplete host-derived ILCs (and not donor cells), recipient mice were treated with an anti-Thy1.2 antibody or an anti-isotype antibody prior to BMT. Pre-transplant depletion of ILCs induced significantly worse systemic signs of GVHD, as well as increased thymic injury and intestinal GVHD pathology, indicating an important role for endogenous ILCs in tissue protection after allo-BMT in this context.
To better understand the roles of tissue-resident ILCs in reducing pathology post-BMT, we evaluated ILC subsets in different GVHD target organs after allo-BMT. Two weeks after MHC-mismatched (C57BL6 into BALB/c) TCD-BMT, both donor- and host-derived ILCs could be detected in the liver, lungs, and intestines. Liver was characterized by a high number of NK cells and ILC1s, while lungs contained a high proportion of ILC2s. The small intestine contained a relatively greater proportion of ILC3s. For all tissues analyzed, the majority of ILCs were host-derived.
During T cell replete BMT resulting in GVHD, both donor and host-derived ILCs were significantly reduced in all three tissues compared to TCD-BMT mice, and this was true for all ILC lineages. To better understand the loss of donor-derived ILCs in GVHD, we investigated ILC precursors in recipient marrow post-BMT, hypothesizing that an impairment of ILC precursors could explain the loss of all ILC lineages in the various tissues analyzed. Indeed, we found a significant loss of CLPs, αLPs, CHILPs, and ILC2Ps in mice with GVHD, suggesting that bone marrow involvement with GVHD induced a loss of ILC precursors impairing their reconstitution.
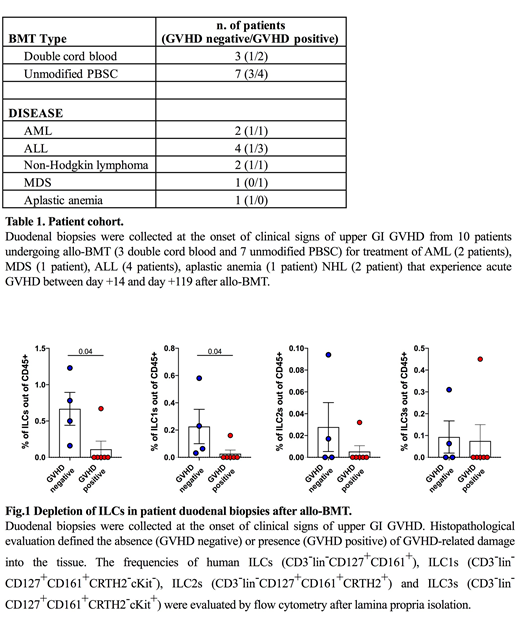
We next sought to evaluate if the loss of tissue-resident ILCs in GVHD target organs after experimental BMT was relevant for clinical transplantation. To evaluate ILC frequencies in patient tissues post-transplant, duodenal biopsies were collected from patients undergoing clinically indicated endoscopy to work up symptoms of acute upper GI (UGI) GVHD. In total, duodenal samples were collected from ten patients post-transplant and 6/10 had histologic evidence of GVHD on biopsy (Table 1). While frequencies were low, ILCs could be identified by FACS in digested lamina propria of duodenal biopsy specimens. Notably, ILC frequencies were significantly lower in patients with biopsy-proven UGI GVHD, and duodenal ILCs could be identified in only 1/6 patients with histologic evidence of GVHD (Fig 1). Among the ILCs that could be identified, ILC1s represented the greatest proportion of ILC populations within the duodenal lamina propria. Accordingly, the frequency of ILC1s was significantly reduced in transplant patients with biopsy-proven UGI GVHD. Additionally, we were able to evaluate paired peripheral blood and duodenal biopsy samples in a small subset of patients. Although the numbers were limiting, this cohort suggested that ILC2s may represent a greater proportion of ILCs in peripheral blood than in the duodenum post-transplant. Overall, clinical findings were consistent with experimental models demonstrating a reduction of ILCs in GVHD.
In conclusion, tissue-resident ILCs contribute to tissue protection after allo-BMT, but GVHD leads to elimination of pre-existing ILCs and loss of ILC precursors in the marrow, impairing their reconstitution. Most duodenal ILCs in clinical biopsy samples post-transplant were ILC1s in this patient cohort, and patients with biopsy-proven UGI GVHD demonstrated significant loss of the few lamina propria ILCs that could be identified in clinical duodenal biopsies.
Hanash:Nexus Global Group LLC: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal