Introduction: Multiple genetic alterations that occur at diagnosis or relapse are not only prognostic characteristics of multiple myeloma (MM) but also provide evidence for clonal evolution. Uncovering and dissecting true driver events in MM might provide rational for new potential targets and therapeutic approaches. However, whereas genetic diagnostics in MM namely FISH and gene expression profiling are well-established prognostic tools, individual mutation profiling has not yet been adopted for this purpose. Herein, we aimed to analyze the Next Generation Sequencing (NGS) platform results investigating mutational profiling of patients with relapsed and refractory MM (RRMM). Also, the clinical results of those who had a targetable mutation and were treated "off label" will be presented.
Material and Methods: A total of 14 consecutive patients with MM referred to our center between November 2018 and May 2019 were studied. Plasma cells were isolated from bone marrow samples using Selection Kit microbead specific for EasySepTM Human CD138 marker (StemCell Technologies). DNA extracted form magnetic bead enriched cells, bone marrow aspiration smears for bone marrow involved, from FFPE tissue samples for extramedullary-involved cases. NGS method was performed on llumina Miseq platform (USA) by using QIAseq targeted DNA panel (12)- Human myeloid neoplasm panel, covers all exons and exon-intron junctions of 141 target genes. For the data analysis QCI Analyze Universal 1,5.0 was performed. The PCL analysis was performed on CD138 and Ki67 double immune stained paraffin sections, and the quantification was done by using 3DHistech digital pathology platform.
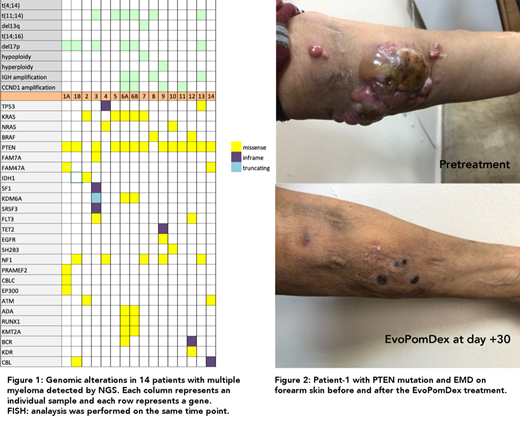
Results: We obtained 16 samples of DNA from 12 heavily pretreated and two newly diagnosed myeloma patients. Female/male: 5/9 with a median age of 57 years (range, 39-87) patients had received a median four lines (range; 1-13) of treatment. Out of a panel of 141 genes, 59 mutations in 26 genes were detected (Figure-1). Among these recurrent genomic abnormalities, concomitant missense protein coding alterations were detected in all patients. The PTEN mutation was the most frequently detected, followed by mutations of RAS/MAPK pathway genes. The hotspots of mutation in KRAS codon 61 and NRAS included codons 61 and 13 as well as codon 600 in BRAF. In addition, we detected novel ie myeloproliferative and myelodysplasia associated mutations previously un-described in myeloma. A diverse range of recurrent gains and losses were detected in our cohort. Two patients at diagnosis also carried mutations of PTEN and KRAS.
Based on these results three patients were able to obtain off-label approval for treatment with Everolimus (for PTEN) (Patient-1) or Trametinib (for KRAS) (Patient 5 & 6) in combination with Pomalidomide (EvoPomDex) w/wo Daratumumab or Tra-PomDex.
Patient-1 had extensive extramedullary disease (EMD) in the skin, which responded completely to Dara-EvoPomDex combination. Complete disappearance of initial lesions (presented in Figure-2) and VGPR duration was only two months. Subsequent refractoriness and appearance of new lesions lead to death of the patient, one year from the initiation of EMD.
Patient-6, also presented with EMD, was treated with TraPomDex as the seventh treatment line. TraPomDex treatment was well tolerated, the most significant adverse event diarrhea, infections and pancytopenia. Her biochemical response was a transient VGPR, which was lost during interruption of treatment due to infection. She also died four months after initiation of TraPomDex. Patient-5, plasma cell leukemia, has been on Tra-PomDex for a month and his response is PR yet.
Conclusion: The detection of mutations can improve our ability to treat multiple refractory patients who have ran out of all therapeutic options. Though the responses observed among such very heavily pretreated patients are not durable, they are highly promising. Also, of additional importance is detection of age-related cumulative mutations belonging to background bone marrow precursors. Detection of sub-clonal mutations is very helpful in depth analysis of clonal response to treatment and clonal evolution. In the coming years, the identification of actionable mutations in myeloma opens the way for targeted therapy.
Acknowledgement:This study is supported by Ankara University Research Grants (Project: 14A0230003) and Turkish Academy of Sciences.
Ozcan:Takeda: Honoraria, Other: Travel support, Research Funding; Bayer: Research Funding; AbbVie: Other: Travel support, Research Funding; Sanofi: Other: Travel support; Abdi Ibrahim: Other: Travel support; Celgene Corporation: Other: Travel support, Research Funding; Janssen: Other: Travel support, Research Funding; Archigen: Research Funding; Roche: Other: Travel support, Research Funding; Jazz: Other: Travel support; MSD: Research Funding; Novartis: Research Funding; Amgen: Honoraria, Other: Travel support; BMS: Other: Travel support. Beksac:Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Speakers Bureau; Janssen: Research Funding, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
trametinib and everolimus for myeloma patients
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal