Background:
Minimal Residual Disease (MRD) was clearly demonstrated as a prognostic marker for prognosis in multiple myeloma and has been integrated into IMWG clinical guidelines as one of the response criteria. There is however no consensus yet when and how to use MRD data in clinical practice throughout treatment course.
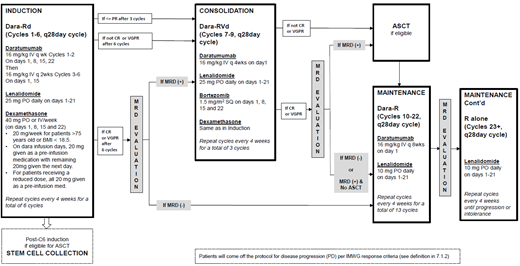
Daratumumab was approved by FDA in different settings after it had been shown as a highly effective agent with a reasonable safety profile in myeloma treatment. It also showed impressive data to achieve MRD negativity. We plan to use the combination of daratumumab, lenalidomide, and dexamethasone upfront, with the addition of Velcade for consolidation if needed, in newly diagnosed multiple myeloma (NDMM) patients.
We propose to use an MRD driven adaptive strategy for decision making in myeloma treatment and use daratumumab-based frontline therapy.
Trial Design and Methods:
We designed this prospective, single-arm, multi-center, phase 2 trial.
The primary objective is to determine the rate of MRD negativity after daratumumab-based induction and consolidation therapy if necessary in both transplant eligible and ineligible NDMM patients.
The secondary objective is to describe the overall survival, progression free survival, MRD conversion rate after consolidation, quality of life for patients, safety and tolerability of daratumumab-based combination regimens in both transplant eligible and ineligible patient populations, estimation of a successful stem cell mobilization after receiving daratumumab-based induction therapy.
The primary endpoint will be MRD-negativity after six cycles of induction, followed by three cycles of consolidation therapy if MRD is positive after induction. We will seek to establish evidence that the true proportion of patients who can achieve MRD negativity after induction + consolidation (if still MRD+ after induction) is high, which we define as any response rate that is at least 23%.
Patients with NDMM meeting IMWG diagnostic criteria are eligible for this trial, regardless of transplant candidacy. Patient will have stem cell collection after induction therapy. We will follow the patients' treatment response using IMWG response criteria including MRD status through induction, consolidation, transplant (if applied) and maintenance. Patient will come off the study if disease progression per IMWG response criteria.
We plan for MRD testing by next generation sequencing and flow cytometry.
This study will enroll 50 eligible patients. A two-stage design will be conducted, with an interim futility analysis after the primary endpoint is available on the first 20 enrolled patients. We will proceed to a final analysis with all 50 patients if at least 2/20 (10%) of patients achieve MRD negativity after consolidation.
This trial is anticipated to be open at four academic centers.
Conclusion:
This multicenter phase 2 trial will apply a novel clinical trial design with an adaptive strategy to treat newly diagnosed myeloma patients based on MRD results.
Therapeutic advance with Daratumumab-based combination regimen as frontline therapy may help myeloma patients to achieve higher rate of MRD negativity.
Ye:AbbVie: Research Funding; Takeda: Research Funding; Celgene: Research Funding; Onyx: Research Funding; Sanofi: Research Funding; Karyopharm: Research Funding; Janssen: Research Funding; MingSight: Research Funding; Portola Pharmaceuticals: Research Funding. Anderson:Amgen, Janssen, Takeda, Celgene: Consultancy, Speakers Bureau. Lipe:amgen: Research Funding; amgen: Consultancy; Celgene: Consultancy. Talpaz:Samus Therapeutics: Research Funding; Imago BioSciences: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; CTI BioPharma: Research Funding; Constellation: Research Funding; Incyte: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal