
Background: Elotuzumab (elo), an immunostimulatory monoclonal antibody targeting signaling lymphocytic activation molecule F7 (SLAMF7, CD319) has shown activity in combination with dexamethasone and lenalidomide (len) or pomalidomide (pom), in patients with relapsed/refractory (RR) multiple myeloma (MM) in the ELOQUENT 2 and 3 trials, respectively. However, patients enrolled on those trials were not refractory to len or pom respectively and the impact of prior daratumumab (dara) (which can deplete natural killer (NK) cells) was not evaluated. We reviewed the outcomes of RR MM patients treated with elo in combination with either len or pom with respect to prior exposure and refractoriness to IMIDs and dara.
Methods: We retrospectively evaluated patients with RR MM who were treated at Moffitt Cancer Center between January, 2017 and July, 2019 with elo in combination with len (elo/len) or pom (elo/pom). We assessed prior therapies and refractoriness to IMIDs and dara. Response was evaluated using the International Myeloma Working Group (IMWG) criteria with added minimal response. Grade 3/4 toxicities were reviewed. This study was approved by the Institutional Review Board.
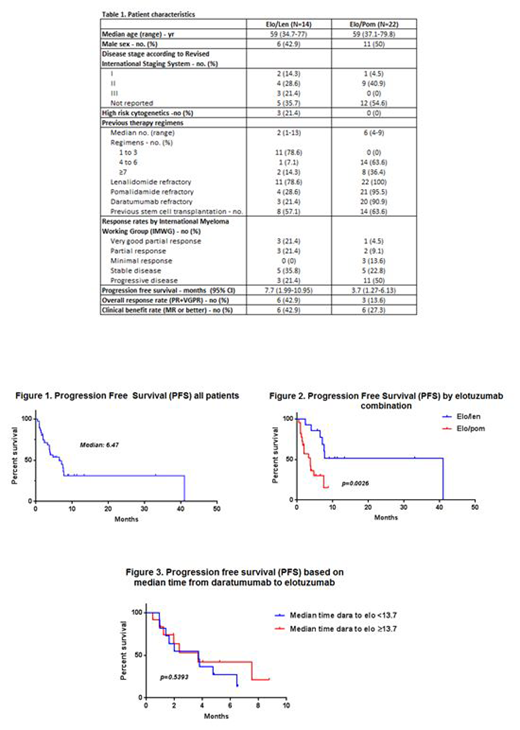
Results: Thirty six patients were identified: 14 on elo/len and 22 on elo/pom. Patient characteristics are outlined in the table. As expected, patients that received elo/pom were more heavily pre-treated, than those who received elo/len, with a median number of 6 prior lines of therapy. The median number of cycles was 9 (range, 2-33) and 3 (range, 1-8) for the elo/len and elo/pom groups, respectively. 21/22 (95%) patients in the elo/pom pts were pom-refractory, whereas 11/14 (79%) of the elo/len patients were len-refractory. For elo/len, the overall response rate (ORR) was 42%, and the median progression-free survival (PFS) was 7.7 months (95% confidence interval, 1.99-10.95, p=0.026) although the ORR was 28.6% in len refractory patients. For elo/pom, the ORR was 14% (for pom refractory patients the ORR was 9.1%) and the median PFS 3.7 months (95% confidence interval, 1.27-6.13 p=0.026). Twenty-three patients (64%) were refractory to dara, with a median time from dara to elo of 13.7 (range, 0.7-33.6) months. Overall survival (OS) and PFS based on the median time from dara to elo were not statistically different. The same analysis based on a 6 months cutoff (assuming shorter dara impact on NK cells) yielded similar results. Thirteen patients remain on treatment at the time of this analysis. Reasons for treatment discontinuation were progressive disease (PD) in 22 (95.7%), adverse event (AE) in 1 (4.3%). Grade 3/4 toxicities were noted in two patients; one patient developed pneumonitis, and another developed infectious complication.
Conclusion: The combination of elo with an IMID, such as len or pom, is well tolerated. Elo/IMIDs regimen have modest efficacy in len and pom refractory patients respectively. The timing of and prior dara does not appear to impact the efficacy of elo based therapy. Prospective studies are needed to confirm these findings.
Shain:Adaptive Biotechnologies: Consultancy; AbbVie: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Brayer:Janssen: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau. Nishihori:Novartis: Research Funding; Karyopharm: Research Funding. Alsina:Bristol-Myers Squibb: Research Funding; Janssen: Speakers Bureau; Amgen: Speakers Bureau. Baz:AbbVie: Research Funding; Merck: Research Funding; Sanofi: Research Funding; Bristol-Myers Squibb: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal