Background: Despite treatment advances, multiple myeloma (MM) remains a condition with high unmet medical need as patients (pts) relapse and become resistant to therapy. MM pts experience a range of serious disease-related symptoms and side effects impacting their functioning and health-related quality of life (HRQL). The median overall survival (OS) has improved with the advent of new treatments in the last two decades, however, the impact of newer combination treatments on pt quality of life remains unknown (Beckner, N et al. Cancer Res. 2011;183:25; Bharat, N et al. J Clin Oncol 2019;37:15(suppl):8039). Venetoclax is a novel, orally bioavailable small molecule inhibitor of BCL-2 activity in MM. In the Phase 3 double-blind, randomized clinical trial (M14-031, BELLINI) progression free survival (PFS) was studied in relapsed/refractory (RR) MM pts receiving venetoclax or placebo in combination with bortezomib and dexamethasone. In addition, patient-reported outcomes (PRO) data focused on physical functioning, pain, and fatigue from BELLINI were evaluated to better understand the overall impact on pt HRQL of adding venetoclax to bortezomib and dexamethasone-based doublet therapy.
Methods: The BELLINI Phase 3 study (NCT01794520) enrolled patients ≥18 y old with R/R MM who had received 1-3 prior lines of therapy. The study met its primary endpoint of PFS (HR 0.63, 95% CI=0.44-0.89) but higher risk of death (HR 2.027, 95% CI=1.04-3.95) was observed in the treatment arm compared with placebo, although the OS data are not yet mature (Kumar, S et al. EHA Library. Jun 16 2019;273254:LB2601). The secondary HRQL endpoints included in this study were pain severity, fatigue, physical functioning (PF), and global health status, which were measured using the Brief Pain Inventory-Short Form (BPI-SF), PROMIS Fatigue Short Form surveys, and the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30). Change from baseline to day 1 of each cycle visit (up to 25 cycles or last treatment visit) was assessed for each measure in both the treatment and placebo groups. Summary analyses compared mean and median scores between arms at each cycle. The treatment effect was evaluated in a mixed effect model controlling for the patient's baseline score, total visits (or cycles of treatment), age (<65 or ≥65), prior therapy lines, and prior proteasome inhibitor exposure. The model also included an interaction term examining treatment by visits.
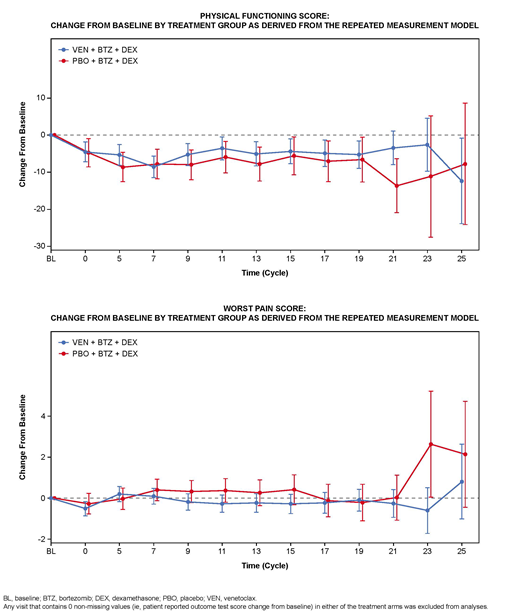
Results: The intention-to-treat (ITT) dataset included 194 pts (median age: 66y; 53% with 2 or 3 prior lines of therapy) in the treatment group and 97 pts (median age: 65y; 55% with 2 or 3 prior lines of therapy) in the placebo group (2:1 randomization). There was no clear evidence for an independent effect of treatment on PF (P=0.522). Pain scores across multiple measures showed a similar trend, with a statistically non-significant improvement of unadjusted average "Worst Pain" scores for the venetoclax group (P=0.289; Figure B). Non-inferiority in pain results were replicated by the pain subscale of the QLQ-30 (P=0.778), the BPI pain severity index (P=0.418), and the related BPI pain interference subscale score (P=0.603). Fatigue scores likewise demonstrated no significant differences between treatment arms whether using PROMIS Cancer Fatigue t-scores (P=0.442), raw scores (P=0.568), or the 3-item QLQ-C30 subscale (P=0.959), although there was a positive trend across cycle summary scores for the treatment group compared with the placebo group. Unadjusted QLQ-C30 PF subscale scores showed declines from baseline that were generally worse in the placebo group across 25 treatment cycles, although differences were smaller and non-significant in the adjusted model (Figure A).
Conclusions: Comparable HRQL in pain severity, fatigue, and PF was observed in RRMM pts receiving venetoclax in combination with bortezomib and dexamethasone compared to pts receiving bortezomib and dexamethasone. The summary scores showed outcomes that were comparable or slightly improved for those randomized to the venetoclax combination. Results of concurrent measures of the same or associated concepts reinforced confidence in these results. Additional analyses of subgroups are ongoing (eg, t(11;14), BCL-2 expression status) and may provide further insights surrounding HRQL in these populations.
Gasparetto:BMS: Consultancy, Honoraria, Other: Travel, accommodations, or other expenses paid or reimbursed ; Celgene: Consultancy, Honoraria, Other: Travel, accommodations, or other expenses paid or reimbursed ; Janssen: Consultancy, Honoraria, Other: Travel, accommodations, or other expenses paid or reimbursed . Popat:Takeda: Honoraria, Other: travel, accommodations, expenses; GSK: Consultancy, Honoraria; Celgene Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations, expenses; Janssen: Honoraria, Other: travel support to meetings; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kumar:Celgene: Consultancy, Research Funding; Takeda: Research Funding; Janssen: Consultancy, Research Funding. Cavo:celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; novartis: Honoraria; sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; bms: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. de la Rubia:Janssen: Consultancy; AbbVie: Consultancy; Celgene: Consultancy; Amgen: Consultancy; Takeda: Consultancy. Hungria:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Salwender:Sanofi: Honoraria, Other: Travel or accommodations; Amgen: Honoraria, Other: Travel or accommodations; Janssen Cilag: Honoraria, Other: Travel or accommodations; AbbVie: Honoraria; Bristol-Myers Squibb: Honoraria, Other: Travel or accommodations; Celgene: Honoraria, Other: Travel or accommodations; Takeda: Honoraria, Other: Travel or accommodations. Suzuki:Ono: Research Funding; BMS: Honoraria, Research Funding; Takeda: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Maciel:Amgen: Other: funding for travel, teaching, participation in advisory boards; Janssen: Other: funding for travel, teaching, participation in advisory boards. Moreau:Takeda: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Harrison:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: investigator on studies, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Jia:AbbVie: Employment, Other: Stock/stock options. Karve:AbbVie: Employment, Other: stock/stock options. Ma:Genentech: Employment, Other: stock options; AbbVie: Other: Alliance Partner. Devine:Genentech: Employment, Other: stock options; AbbVie: Other: Alliance Partner. Benjamin:AbbVie: Employment, Other: stock/stock options.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal