ABSTRACT
BACKGROUND
Daratumumab is an anti-CD38 monoclonal antibody that is used more frequently in patients with Multiple Myeloma (MM). Multiple studies have demonstrated the efficacy and safety of daratumumab for relapsed/refractory MM and primary treatment for transplant eligible and ineligible patients.
METHODS
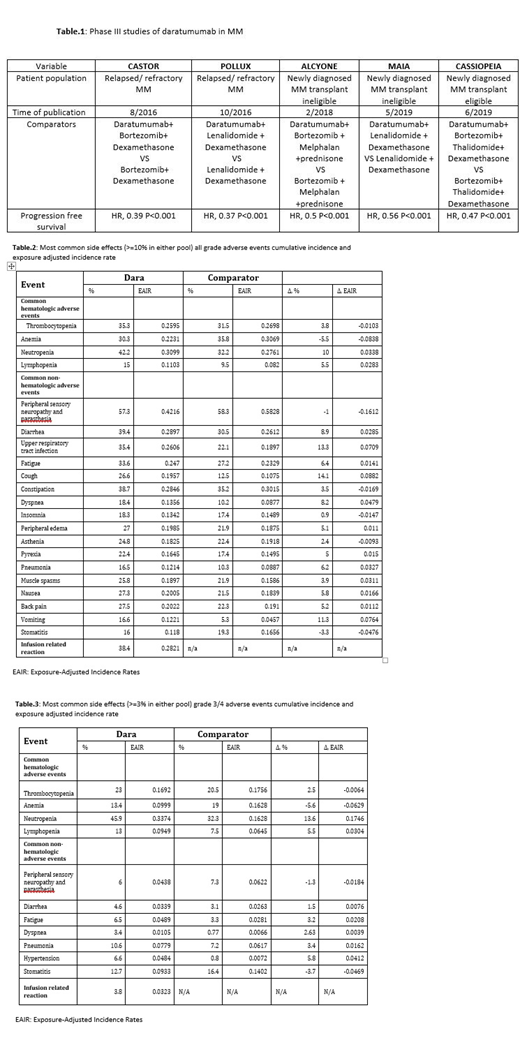
We conducted an integrated safety analysis to characterize the frequency, severity, natural history, and outcomes of adverse events (AEs) with daratumumab versus comparators. Data were pooled from 5 completed phase III randomized controlled studies that had included 1798 daratumumab-treated and 1797 comparator-treated patients with MM as a first line in both transplant eligible and transplant ineligible and for relapsed/refractory disease(Table.1). Safety analyses included reporting of AEs using crude and exposure-adjusted incidence rates.
RESULTS
The median follow up duration is 16.84 months (range: 7.4-28 months) for both daratumumab-treated and comparator-treated patients. Discontinuation for any reason occurred less often with daratumumab (22% vs. 33.9%) though discontinuation because of AEs occurred at similar rates (25% vs.26%) as well as deaths due to AEs occurred at similar rates (2.25% vs. 1.84%). When adjusted to exposure, neutropenia, lymphopenia, diarrhea, fatigue, dyspnea, pneumonia and hypertension were the only common grade 3 AEs more often reported with daratumumab than with the comparators(Table.2). The prevalence of common grade 3/4 AEs with daratumumab were <7% apart from neutropenia, lymphopenia and pneumonia (45.9 vs. 32.3, 13 vs. 7.5, and 10.6 vs.7.2)(Table 3). Grade 3/4 daratumumab infusion related reactions happened in 3.8%
CONCLUSIONS
These results from an integrated analysis support a favorable benefit/risk profile of daratumumab in patients with MM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal