Background: triplet combinations comprising a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD) are current standard induction and consolidation regimens in NDMM. The all-oral combination of weekly ixazomib plus lenalidomide-dexamethasone (IRd) has been evaluated by several groups in NDMM and is approved in relapsed-refractory MM. The IFM 2014-01 phase 2 trial previously studied the weekly IRd regimen as induction and extended consolidation followed by single-agent ixazomib maintenance in frontline transplant eligible patients (Moreau et al ASH meeting 2016): IRd was well tolerated and overall response rate was 81%, including 38% very good partial response or better (≥VGPR) at the completion of induction (3 cycles). Responses further increased at each step of the program and 76% of patients (per protocole analysis) achieved ≥VGPR before maintenance with 6% CR and 38% sCR. To stay in line with current RVd regimen, and to increase dose intensity, we examined the efficacy and safety of twice-weekly ixazomib +Rd as induction prior to transplant, followed by weekly IRd consolidation and single-agent lenalidomide maintenance (NCT02897830).
Methods: This is a phase II, single-arm, open-label, multicenter study. During induction, patients received three 21-day cycles of twice-weekly oral IRd: ixazomib (3 mg on days 1, 4, 8 and 11), lenalidomide (25 mg daily, days 1-14), and dexamethasone (40 mg on days 1, 4, 8 and 11) followed by transplant. Patients then received two 28-day cycles of weekly IRd early consolidation followed by 6 additional cycles of IR (no dexamethasone) as late consolidation (ixazomib 4mg on days 1-8 and 15; lenalidomide 25mg daily, days 1-21). Single-agent lenalidomide maintenance was administered for up to 1 year (10 mg daily, days 1-21). The primary endpoint was the stringent complete response (sCR) rate at the completion of consolidation. The secondary endpoints included assessments of overall response rate (ORR) and rates of response categories at each step of the program, progression-free survival (PFS), feasibility and safety. Responses were assessed in accordance with the IMWG uniform criteria. Toxicity was evaluated according to NCI CTCAE, version 4.03.
Results
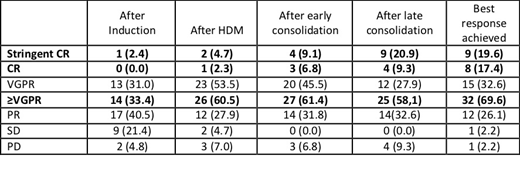
Between 07/2016 and 08/2017, 50 patients with NDMM were screened at 10 IFM centers, 46 were enrolled with a median age of 59 years, and 59% were male. The percentages of patients with ISS stage I, II, and III were 41.5%, 41.5%, and 17%, respectively. High-risk cytogenetics, defined as t (4; 14), or del17p (central Lab, H. Avet-Loiseau), was observed in 9% of patients (6.5% FISH failure). As of July 1st 2019 (data cut-off), 10 patients prematurely discontinued therapy. Considering efficacy, 43/46 patients (94%) completed consolidation and 9 achieved sCR (20.9%; 90% CI [11.4 to 33.7]). This result did not meet the minimum efficacy threshold (40%) for the primary efficacy endpoint (p=0.998). Overall, at the completion of consolidation, ORR was 91% including 21% sCR, 30% ≥CR and 58%≥VGPR. Responses at each step of the program are described in the table 1. If we focus on twice-weekly IRd induction, at the completion of 3 cycles, ORR was 74%, including 33% ≥VGPR. The feasibility of the program was good and overall, 39/46 patients (85%) were able to receive maintenance therapy with single-agent lenalidomide. After a median follow-up of 22 months, 7 patients progressed and 3 patients died. Concerning safety: 31 serious treatment emergent AEs were reported in 20 patients (43.5%) comprising infections (8 patients), cardiac disorders (2 patients: ischemic heart disease and aortic valve incompetence), psychiatric, renal and respiratory disorders (2 cases each). No grade 3-4 peripheral neuropathy was described.
Conclusions
The all-oral Ixazomib-Lenalidomide-Dexamethasone (IRd) induction/consolidation regimen in the transplant setting is convenient, well tolerated, leading to 21% sCR before maintenance. Twice-weekly IRd induction does not seem superior to weekly IRd induction Results on response rates following maintenance and MRD data will be presented during the meeting.
Roussel:Celgene Corporation: Consultancy, Other: travel fees, lecture fees, Research Funding; takeda: Other: travel fees, lecture fees, Research Funding; Amgen: Other: travel fees, lecture fees, Research Funding; Janssen: Honoraria, Other: travel fees, lecture fees, Research Funding. Hebraud:celgene: Other: travel fees, lecture fees; takeda: Other: travel fees, lecture fees. Hulin:Janssen, AbbVie, Celgene, Amgen: Honoraria; celgene: Consultancy, Honoraria. Leleu:Oncopeptide: Honoraria; Sanofi: Honoraria; Takeda: Honoraria; Karyopharm: Honoraria; Amgen: Honoraria; Carsgen: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Janssen: Honoraria; BMS: Honoraria; Merck: Honoraria. Facon:Amgen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Touzeau:celgene: Other: travel fees, lecture fees, Research Funding; takeda: Other: travel fees, lecture fees. Perrot:jannsen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; takeda: Honoraria. Stoppa:celgene: Other: travel fees, lecture fees; takeda: Other: travel fees. Moreau:Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Avet-Loiseau:takeda: Consultancy, Other: travel fees, lecture fees, Research Funding; celgene: Consultancy, Other: travel fees, lecture fees, Research Funding. Attal:celgene: Consultancy, Other: travel fees, lecture fees, Research Funding; takeda: Consultancy, Other: travel fees, lecture fees, Research Funding.
Ixazomib is indicated in RRMM in association with Rd
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal