Recent advances of monoclonal antibodies provide a potent anti-myeloma effect based on antibody-dependent cytotoxicity (ADCC). Elotuzumab is a monoclonal antibody which binds SLAMF7 on the surface of myeloma cells and induces ADCC by NK cells (Cancer Immunol Immunother 64:61-73,2015). Lenalidomide may enhance the killing activity ad IFN-gamma production in NK cells (Blood 126:50-60,2015). In Eloquent-2 trial, the combination therapy of Elotuzumab, Lenalidomide, and Dexamethasone (ELD) showed a significant relative reduction in the risk of disease progression or death (N Engl J Med 373:621-631,2015). However, the number of NK cells in patients with multiple myeloma is generally suppressed, and the activity is often exhausted (Cytometry 26:121-124,1996, Med Oncol 24:312-317,2007). It is unclear if they fully exhibit the ADCC against myeloma cells. Also, enough number of NK cells as effector cells is required to obtain effective ADCC.
To enhance the ADCC activity of NK cells against multiple myeloma, cell therapy using expanded NK cells might be effective.
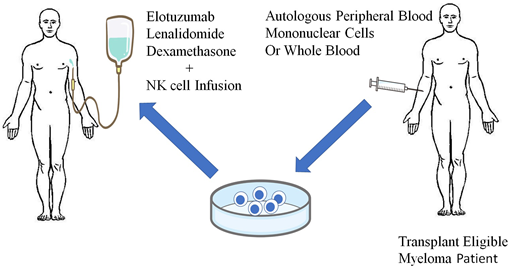
Therefore, we conduct the clinical trial of ex-vivo expanded NK cell infusion therapy in combination with ELD for minimal residual disease (MRD) after autologous stem cell transplantation (ASCT).
Clinical trial registry number: UMIN000033128
Study design: Non-randomized single-arm phase I/ II clinical trial.
Study population: 20; Five for phase I part and 15 for phase II.
Primary endpoint: Any grade of adverse events, and the response defined by IMWG criteria.
Secondary endpoint: Overall survival, progression-free survival, and MRD.
Study protocol: 1x106 - 5x107/kg of ex-vivo expanded NK cells are infused on day 2 of ELD regimen in patients who failed to achieve sCR or with detective MRD after ASCT. The NK cell infusion with ELD therapy will be repeated 4 cycles monthly.
Major inclusion criteria: (1)Multiple myeloma diagnosed by IMWG diagnostic criteria, (2)Age: 20=<, 65>=, (3)ECOG Performance status0-2, (4)PR or better after induction therapy, (5) Transplant eligible patients without severe organ damages, (6)Patients failed to achieve sCR after stem cell transplantation or positive for minimal residual disease by 6-8 color Flow cytometry.
Major exclusion criteria: (1) Patients who were previously transplanted, (2) Failed to obtain more than 2x106/kg of CD34+ cells, (3) Patients who are not eligible for harvest or transplant due to cardiac or pulmonary disorder, AST/ALT/total bilirubin>2.5x lower limit, serum Cr>=2.0mg/dl, (4) Patients who have severe allergy or uncontrollable active infection.
Currently, two participants completed the protocol and are under the observation.
Hagiwara:Bristol-Myers Squibb: Research Funding. Kanno:Agios Pharmaceuticals, Inc.: Honoraria. Tanaka:Bristol-Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal