Background
Early trials of long-term lenalidomide (len) use reported an increased incidence of second primary malignancy (SPM), particularly AML and MDS. Later, meta-analysis suggested the link to be secondary to len in combination with melphalan. Myeloma XI is a large phase III randomised trial in-which len is used as an induction and maintenance therapy. We have previously reported on early SPM incidence in the context of this trial and shown that haematological SPM (hSPM) incidence was not increased in association with len (Cumulative incidence 0.7% at 3 years). We have also published data showing that maintenance len is associated with improved survival in post-transplant patients. Here we present for the first time, a long-term analysis of SPM incidence in 4358 patients who have received maintenance therapy versus observation in the context of the Myeloma XI trial.
Methods
Myeloma XI is a phase III, randomised, multi-centre, parallel group design, open-label trial comparing thalidomide, len, carfilzomib and bortezomib combinations and len as maintenance treatment in NDMM patients. The trial includes transplant eligible (TE) and transplant non-eligible (TNE) pathways. TE patients received high dose melphalan supported by autologous stem cell transplantation if they achieve a very good partial response or better. Patients in both pathways were randomised to maintenance with len (+/- vorinostat) or active observation. Since May 2010, 4358 patients have been treated and assigned to induction. Median follow up is now 68 months (IQ range 49-83), with over 1100 patients enrolled for more than 5 years. A total of 1971 patients have entered maintenance with 1137 randomised to len and 834 observation (median 24 cycles (range 1-97)).
Results
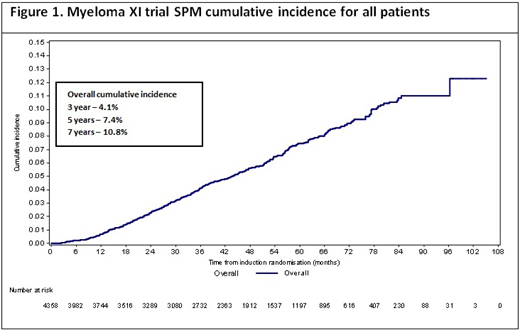
The median time to SPM development from induction is 41.6 months (range 1.4 - 97.1). Cumulative incidence of all SPMs is 4.1% (95% CI 3.5, 4.7), 7.4% (6.6, 8.3) and 10.8% (9.5, 12.1) at three, five and seven years respectively (figure 1).
378 SPMs have been reported in 318 patients, 180 (57%) treated in the TNE pathway (IR 9.9%) and 138 (43%) in the TE pathway (IR 5.5%). The average age at the time of SPM is 78 and 68 for the TNE and TE pathways, respectively.
Of the patients who developed an SPM 195 (61%) did so following maintenance randomisation. The incidence was highest in those receiving len based maintenance (n=145, IR 10.8%). In the observation arm 50 patients developed an SPM (IR 5.6%). The median time to SPM development from maintenance randomisation was 43 months in those receiving len and 40 months in the observation only group.
One hundred and ten of the patients who developed an SPM following maintenance randomisation were in the TNE pathway with 77 patients receiving len. The overall incidence of SPM development in TNE patients receiving len maintenance is 15.4% and 9.9% for observation. Within the TE arm 85 SPM cases developed following maintenance randomisation with 68 patients receiving len. The overall incidence of SPM development in the TE patients treated with len maintenance is 7.7% and 3.1% for observation.
Of the remaining patients 98, (31%) developed an SPM during induction and twenty five (8%) following induction but prior to maintenance.
Forty eight patients developed a hSPM (IR 1.1%), MDS (n=20), AML (n=13), BLCBL (n=6), B-ALL (n=4), CML (n=1), mixed phenotype leukaemia (n=1), T-ALL (n=1), Burkitt lymphoma (n=1) and Hodgkin's Lymphoma (n=1). Of these cases, 35 patients were TE pathway treated, with 25 receiving lenlidomide maintenance (IR 2.8%). The median time to hSPM development in the TE patients was 51.8 months. The remaining 13 cases occurred in the TNE treated patients with 7 receiving lenalidomide maintenance (IR 1.4%). The median time to hSPM development in the TNE patients was 42.3 months.
Of the remaining cases, 107 patients developed non-melanoma skin cancers (NMSC) (TE n34, IR 1.3% / TNE n73, IR 4.0%) and 181 patients developed solid tumours (TE n78, IR 3.1% / TNE n103, IR 5.6%). Forty one patients developed >1 SPM. Outcome analysis is underway and will be presented at the meeting.
Conclusions
The overall incidence of SPM is highest in TNE patients receiving lenalidomide maintenance but hSPM rates remain low. This suggests that advanced age is a considerable risk factor for the development of second cancers, particularly NMSC. Second haematological cancers were low in all groups, irrespective of maintenance allocation.
Jones:Celgene: Honoraria, Research Funding. Cairns:Celgene, Amgen, Merck, Takeda: Other: Research Funding to Institution. Pawlyn:Amgen, Celgene, Takeda: Consultancy; Amgen, Celgene, Janssen, Oncopeptides: Honoraria; Amgen, Janssen, Celgene, Takeda: Other: Travel expenses. Davies:Amgen, Celgene, Janssen, Oncopeptides, Roche, Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Consultant/Advisor; Janssen, Celgene: Other: Research Grant, Research Funding. Jenner:Abbvie, Amgen, Celgene, Novartis, Janssen, Sanofi Genzyme, Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kaiser:Takeda, Janssen, Celgene, Amgen: Honoraria, Other: Travel Expenses; Celgene, Janssen: Research Funding; Abbvie, Celgene, Takeda, Janssen, Amgen, Abbvie, Karyopharm: Consultancy. Mottram:Celgene, Amgen, Merck, Takeda: Other: Research Funding to Institution. Drayson:Abingdon Health: Consultancy, Equity Ownership. Cook:Celgene, Janssen-Cilag, Takeda: Honoraria, Research Funding; Amgen, Bristol-Myers Squib, GlycoMimetics, Seattle Genetics, Sanofi: Honoraria; Janssen, Takeda, Sanofi, Karyopharm, Celgene: Consultancy, Honoraria, Speakers Bureau. Gregory:Amgen, Merck: Research Funding; Abbvie, Janssen: Honoraria; Celgene: Consultancy, Research Funding. Owen:Celgene, Janssen: Honoraria; Celgene, Janssen: Consultancy; Celgene: Research Funding; Janssen: Other: Travel expenses. Boyd:Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Jackson:Celgene, Amgen, Roche, Janssen, Sanofi: Honoraria. Morgan:Celgene Corporation, Janssen: Research Funding; Bristol-Myers Squibb, Celgene Corporation, Takeda: Consultancy, Honoraria; Amgen, Janssen, Takeda, Celgene Corporation: Other: Travel expenses.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal