Background: Owing to the development of novel agents, the rate of complete response (CR) in multiple myeloma (MM) has increased. Additionally, the development of methods for measuring minimal residual disease (MRD) (e.g., multiparameter flow cytometry [MFC] and next-generation sequencing) has enabled us to stratify CR patients according to MRD levels. In this study, we hypothesized that deep response predicts better prognosis in MM. To investigate this hypothesis, we assessed the response of patients treated with carfilzomib + lenalidomide + dexamethasone (KRD) using MFC and compared survival outcomes between different groups defined by the MRD status.
Methods: The response of patients with relapsed/refractory MM treated with KRD at four different centers between September 2016 and October 2018 was prospectively investigated using the EuroFlow next-generation flow (EuroFlow-NGF) method. In this method, ammonium chloride-based bulk lysis was used, followed by surface staining with antibodies against CD138-BV421, CD27-BV510, CD38 multiepitope (ME)-FITC, CD56-PE, CD45-PerCP Cy5.5, CD19-PECy7, CD117-APC, and CD81-APC C750 in tube 1 and surface/intracellular staining with antibodies against CD138-BV421, CD27-BV510, CD38 ME-FITC, CD56-PE, CD45-PerCP Cy5.5, CD19-PECy7, CD117-APC, CD81-APC C750, cytoplasmic (cy) Igκ-APC, and cyIgλ-APC C750 after permeabilization in tube 2. MRD levels were assessed using bone marrow (BM) cells after several KRD cycles, with the lower limit of detection set at 1 × 10−5. Presence of high-risk cytogenetics [del 17p, t(4;14) and/or t(14;16)] in BM cells was analyzed through FISH.
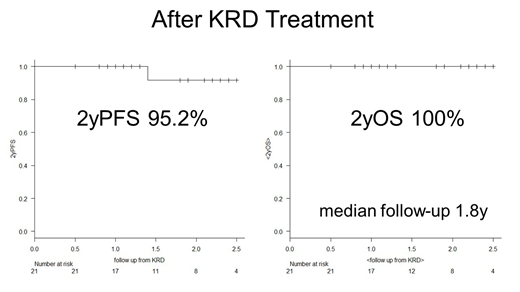
Results: A total of 21 patients (12 males, 9 females) were treated with KRD and assessed for MRD levels. The median age of these patients was 66 years at KRD initiation (range 30-83 years), and 11 patients had ISS 1, 6 had ISS 2, and 4 had ISS 3. Four patients displayed high-risk chromosomal abnormalities, including del 17p (n = 3) and t(14;16) (n = 1). The median number of prior treatments was 3 (range 1-6); these included bortezomib (n=12), lenalidomide (n=19), and autologous stem-cell transplantation (n=12). The median number of KRD cycles was 4 (range 1-22). The proportion of patients achieving ≥CR and overall response (≥ partial response [PR]) was significantly higher after KRD treatment than the proportion that had been achieved by previous therapies (71% vs. 9.5%, p < 0.001; 100% vs. 71%, p = 0.008, respectively). Pre-KRD responses included 2 stringent CR (sCR), 7 very good PR (VGPR), 6 PR, 3 stable disease, and 3 progressive disease. Post-KRD responses included 13 sCR, 2 CR, 3 VGPR, and 3 PR. A total of 95% (20/21) of patients achieved sCR, and 5% (1/21) VGPR as best response. After KRD, response was upgraded in 19 (90%) patients and maintained in two PR (10%) patients. During and after KRD treatment, MRD negativity was achieved in 12 of 16 (75%) and in 15 of 21 (71%) patients, respectively. The median number of therapy lines after KRD was 1 (range 0-5). All 4 high-risk cytogenetic cases achieved MRD negativity. Among MRD-positive cases, both 2-year progression-free survival (PFS) and 2-year overall survival (OS) from KRD initiation were 100%. Among MRD-negative cases, 2-year PFS and OS from KRD initiation were 92% and 100%, respectively. The median follow-up was 1.8 years (range 0.5-2.5 years). One MRD-negative case showed extramedullary relapse 1.4 years after the last KRD cycle. This patient did not have high-risk cytogenetics and achieved "flow MRD negativity" after two KRD cycles, and the treatment was stopped after 7 KRD cycles due to peripheral neuropathy. Paiva et. al. also reported that only 6 of 225 (3%) MRD-negative patients relapsed. Strikingly, all 6 relapsing cases in the report had extramedullary plasmacytomas at diagnosis; all relapsed with extramedullary plasmacytomas and only 2 developed concomitant serological relapse (ASH 2017, abstract #905).
Conclusions: KRD induced deep responses in relapsed/refractory MM patients who eventually displayed excellent PFS. All patients with high-risk cytogenetics achieved EuroFlow-NGF negativity. Post-remission imaging studies such as MRI/PET-CT may be necessary for patients who presented with extramedullary plasmacytomas even when they achieved flow MRD negativity.
Yoroidaka:Ono Pharmaceutical: Honoraria. Takamatsu:Celgene: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Ono pharmaceutical: Honoraria, Research Funding; CSL Behring: Research Funding; SRL: Consultancy, Research Funding; Janssen Pharmaceutical: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Fujimoto Pharmaceutical: Honoraria; Becton, Dickinson and Company: Honoraria; Abbvie: Consultancy; Daiichi-Sankyo Company: Honoraria. Yamashita:Celgene: Honoraria; Ono Pharmaceutical: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Bristol-Myers Squibb: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Chugai Pharmaceutical Co.,Ltd: Honoraria; Kyowa Kirin: Honoraria; Daiichi-Sankyo Company: Honoraria; TEIJIN PHARMA LIMITED: Honoraria. Murata:Celgene: Honoraria; Ono pharmaceutical: Honoraria. Yoshihara:Kyowa Kirin: Honoraria; Celgene: Honoraria; Bristol-Myers Squibb: Honoraria; ONO PHARMACEUTICAL CO., LTD.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Eisai Co., Ltd.: Honoraria. Yoshihara:Chugai Pharmaceutical Co.,Ltd: Honoraria; Bristol-Myers Squibb: Honoraria; Novartis Pharma K.K.: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Sumitomo Dainippon Pharma: Honoraria; Kyowa Kirin: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Celgene: Honoraria; ONO PHARMACEUTICAL CO., LTD.: Honoraria. Nakao:Bristol-Myers Squibb: Honoraria; Chugai Pharmaceutical Co.,Ltd: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Celgene: Honoraria; Alaxion Pharmaceuticals: Honoraria; Ohtsuka Pharmaceutical: Honoraria; Novartis Pharma K.K: Honoraria; Kyowa Kirin: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Ono Pharmaceutical: Honoraria; Daiichi-Sankyo Company, Limited: Honoraria; SynBio Pharmaceuticals: Consultancy. Matsue:Takeda Pharmaceutical Company Limited: Honoraria; Novartis Pharma K.K: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Celgene: Honoraria; Ono Pharmaceutical: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal