Background: The treatment of Multiple Myeloma (MM) has evolved significantly in the past decade with the introduction of novel agents and drug combinations, thus enhancing treatment efficacy and allowing more patients to achieve complete response (CR). This has created a need to identify surrogates for depth of treatment response. Serum free light chain (sFLC) ratio normalization has been shown to be prognostic for progression free survival as well as overall survival in patients achieving a complete response to therapy. Consequently, it has been incorporated as a defining feature for stringent CR, along with lack of clonal plasma cells by immunohistochemistry (IHC) or low sensitivity flow cytometry. The routine use of multiparametric flow cytometry with higher sensitivity to detect residual disease than IHC or the older 4-color flow cytometry, has raised the question as to whether sFLC ratio is still a valid indicator of response depth. Moreover, in nearly half of the patients with an abnormal sFLC ratio after treatment, the abnormality is secondary to suppression of one or both serum light chains. Therefore, we designed a retrospective study to address these issues.
Patients and Methods: This is a retrospective study using the Multiple Myeloma Database at Mayo Clinic, Rochester. We included patients who, after any line of therapy, had negative serum and urine immunofixation and absence of clonal bone marrow plasma cells by flow cytometry (PC-PRO), which has a sensitivity of >10-4. Simultaneous sFLC data was also extracted. Patients were grouped into three categories based on their sFLC ratios: 1) normal ratio (normal), 2) abnormal ratio due to suppression of the uninvolved light chain (LC), involved LC, or both (Abn-suppressed) and 3) abnormal ratio due to elevation of the involved LC (Abn-inv elevated). The primary endpoint was the median time to next treatment (TTNT), defined as the time from sample collection to the time of initiation of the subsequent therapy or time of last follow up if a subsequent line of treatment was not initiated.
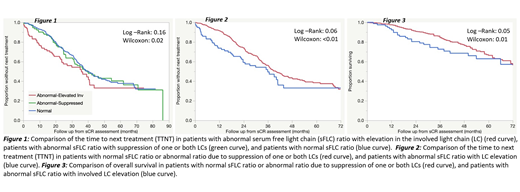
Results: The cohort consisted of 510 patients. 285 (56%) were males and 225 (44%) females. Median age was 61 years (IQR: 55-67). Median Follow-up was 41 months. The last treatments administered prior to data collection included stem cell transplant (SCT) (with or without maintenance) in 290 (57%) patients, and non-SCT regimens in the others. The sFLC ratio was normal in 337 (66%) and abnormal in 173 (34%) patients. Among the patients with abnormal sFLC ratios, 81 had elevated involved LC, 25 had suppression of the involved LC, 45 had suppression of the uninvolved LC and 22 had suppression of both LCs. We first examined the TTNT for the three groups and found that the TTNT was identical for those with a normal ratio and those with an abnormal ratio due to suppression of one or both light chains (Figure 1). So, we combined these two groups (Normal-Abn suppressed) and compared their outcomes to the patients with abnormal sFLC ratio due to elevated involved LC. The Abn-inv elevated group had a shorter TTNT as shown in Figure 2 (log-rank 0.06, Wilcoxon <0.01). The Abn-inv elevated group also had decreased overall survival compared to the other group (log-rank: 0.05, Wilcoxon: 0.01) (Figure 3).
Conclusion: This study provides 2 important observations. First, patients with an abnormal ratio due to suppression of one or both LCs have outcomes similar to those with a normal ratio, suggesting a need to clarify the current definition of stringent CR. Second, the study suggests an important prognostic value for an abnormal sFLC ratio due to elevated involved LC, suggesting this as an important surrogate for depth of response.
Kapoor:Janssen: Research Funding; Takeda: Honoraria, Research Funding; Cellectar: Consultancy; Celgene: Honoraria; Sanofi: Consultancy, Research Funding; Amgen: Research Funding; Glaxo Smith Kline: Research Funding. Dispenzieri:Akcea: Consultancy; Intellia: Consultancy; Janssen: Consultancy; Pfizer: Research Funding; Takeda: Research Funding; Celgene: Research Funding; Alnylam: Research Funding. Gertz:Ionis: Honoraria; Alnylam: Honoraria; Prothena: Honoraria; Celgene: Honoraria; Janssen: Honoraria; Spectrum: Honoraria, Research Funding. Lacy:Celgene: Research Funding. Dingli:Karyopharm: Research Funding; Rigel: Consultancy; Millenium: Consultancy; Janssen: Consultancy; alexion: Consultancy. Leung:Takeda: Research Funding; Aduro: Membership on an entity's Board of Directors or advisory committees; Prothena: Membership on an entity's Board of Directors or advisory committees; Omeros: Research Funding. Kumar:Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal