Targeted drug treatment strategies have significantly prolonged the overall survival rate among multiple myeloma (MM) patients. However, high relapse rates and multiple drug resistance still pose major challenges. Although, the underlying molecular features of the disease have been explored both at the genomic and transcriptomic levels, the functional role of microRNAs (miRNA) in MM disease progression and prognosis is yet to be investigated at a personalized level. In earlier studies, microRNAs have been implicated to regulate gene expression and were determined to play crucial roles in the biology of MM by acting as oncogenes or tumor suppressors. Nevertheless, considering the heterogeneity of MM, little is known about the roles of miRNAs in controlling MM disease progression and drug response at an individualized systems level.
We collected bone marrow aspirates from MM patients at diagnosis (n=20) and relapse (n=25) after informed consent and following approved protocols in accordance with the Declaration of Helsinki. CD138+ plasma cells were enriched from the bone marrow samples and used for miRNA-sequencing and drug sensitivity and resistance testing (DSRT). The miRNA was prepared from the CD138+ cells and subjected to sequencing using Illumina compatible technologies. DSRT was performed and responses to 83 clinically approved drugs and investigational compounds were measured as drug sensitivity scores (DSS) as described previously (Majumder et al., Oncotarget 2017). The pairwise comparative analysis of miRNA expression and drug responses was performed using Spearman's rank-order correlations, to elucidate significant associations of miRNA expression with drug sensitivity and resistance. Additionally, using DEseq2 the differential miRNA expression was determined for the newly diagnosed and relapse samples to deconvolute the role of miRNAs in MM disease progression.
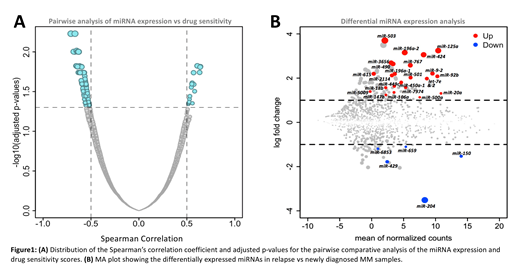
The comparative analysis of the miRNA expression and drug sensitivity scores revealed statistically significant associations between miRNA expression and drug sensitivity measures with the Spearman coefficient (r) ranging from -0.71 to 0.64 (adjusted p-value ≤ 0.05) (Figure 1A). Negative associations were more prevalent, with 40 miRNAs negatively associated with ≥1 drug response from the total of 30 predicted drugs. miR-486, which is known to be an effective biomarker in diagnosis and prognosis of multiple cancer types (Jiang et al., Oncotarget 2018), was found to have significant negative correlation (r= -0.71 to -0.52, p-value ≤ 0.01) with the responses of 14 drugs. Similarly, negative correlation was observed for miR-144 with 12 drugs and miR-584 with 9 drugs. We observed that PI3K/mTOR inhibitors and HDAC inhibitors were common amongst all the significant negative correlations predicted. Specifically, the PI3K/mTOR inhibitors apitosilib, omipalisib and buparlisib were found to be negatively associated with the expression of 18, 14 and 7 miRNAs respectively. These observations can lead to the understanding of miRNA mediated regulation of molecular pathways involved in drug resistance.
Differential miRNA expression analysis between newly diagnosed and relapse MM samples revealed the involvement of miRNAs in disease progression. The analysis resulted in total of 31 significant differentially expressed miRNAs with fold change ≥2 and adjusted p-value ≤ 0.1 (Figure 1B). Several miRNAs known to play crucial roles in cancer diagnosis and prognosis were found to be significantly upregulated in the relapse samples. In particular, 25 miRNAs were upregulated, including following miR-17/92 cluster members: miR-18b, miR-20a, miR-92b and miR-106a, which are known to have an oncogenic role in various cancer types (Mogilyansky & Rigoutsos, Cell Death and Differentiation 2013). Interestingly, 12/31 differentially regulated miRNAs were located on chromosome X. Although cytogenetic alteration data predicted that chromosome 1q gain is significantly prominent in the relapse samples (p-value = 0.009), only 3/31 differentially regulated miRNAs were located on chromosome 1.
These results demonstrate the role of miRNAs in regulating drug response and disease progression in multiple myeloma. Monitoring miRNA expression profiles in MM patients can facilitate the assessment of treatment outcome and prognosis, and miRNAs could potentially be useful prognostic and treatment biomarkers for MM.
Silvennoinen:Amgen: Research Funding; Bristol-Myers Squibb (BMS): Research Funding; Takeda: Research Funding; Celgene: Research Funding. Heckman:Celgene: Research Funding; Novartis: Research Funding; Oncopeptides: Research Funding; Orion Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal