Background: Of the several drugs and drug combinations approved for treatment of relapsed and refractory chronic lymphocytic leukemia (CLL), the reported complete response rates are no greater than 30%. Obinutuzumab is a glycoengineered, humanized type 2 anti-CD20 monoclonal antibody thought to engage the immune system by directly activating antibody- dependent, cell-mediated cytotoxicity (ADCC); it is approved for treatment of chronic lymphocytic leukemia in combination with chlorambucil. The key mediators of ADCC are polymorphonuclear neutrophils, monocytes, and natural killer (NK) cells. Recombinant human Interleukin-15 (rhIL-15) is a stimulatory cytokine that promotes the differentiation and activation of NK cells, monocytes, and long-term CD8+ memory T-cells. In a Phase I trial, administration of rhIL-15 as a 5-day continuous intravenous infusion (civ) was associated with up to 45-fold increase in the number of NK cells at well- tolerated dose levels. Preclinical murine lymphoid malignancy models have shown increased efficacy of monoclonal antibodies when administered together with rhIL-15; BL/6 mice inoculated with EL4-CD20 cells (a syngeneic lymphoma line); including significant prolongation of survival with the IL-15/Rituximab combination compared to either drug given as single agent (90% v. 30% alive at 75 days). We hypothesized that rhIL-15-associated increase in NK cell number and activity would improve efficacy of obinutuzumab in treatment of relapsed and refractory CLL, and would increase the duration and depth of response; this phase I trial is testing the safety of the combination.
Primary objective: determine the safety, toxicity profile, dose-limiting toxicity (DLT) and the maximum tolerated dose (MTD) of civ rhIL-15 administration in combination with obinutuzumab treatment
Secondary objectives: 1) evaluate the potential antitumor activity of the combination of rhIL-15 and obinutuzumab by assessing the clinical response rate, minimal residual disease (MRD) status, progression-free survival, and overall survival in patients with relapsed and refractory CLL; 2)
define the effects of rhIL-15 on the ADCC mediated by obinutuzumab using ex vivo peripheral blood mononuclear cells (PBMCs); 3) characterize the biological effects of rhIL-15 administered with obinutuzumab on the percentages and absolute numbers of circulating lymphocytes (T and NK cells) and the T- cell subsets (including naïve, central, and effector memory subsets) by flow cytometry
Exploratory objectives: identify biomarkers predictive of response to rhIL-15 and obinutuzumab treatment, such as circulating tumor DNA (ctDNA) and baseline cytokine levels
Eligibility criteria: 1) age ≥ 18 years; 2) ECOG ≤ 1; 3) Diagnosis of CLL or small lymphocytic lymphoma (SLL) with ≥ 50% of B cells expressing CD20; 4) measurable or evaluable disease that is refractory or relapsed following therapy with a BTK inhibitor OR have relapsed/refractory CLL and are intolerant to BTK inhibitor therapy; patients with del(17p) must also be refractory or relapsed after, or intolerant to, therapy with venetoclax; 5) adequate organ function parameters as defined within the protocol; 6) active disease requiring treatment, as defined within the protocol.
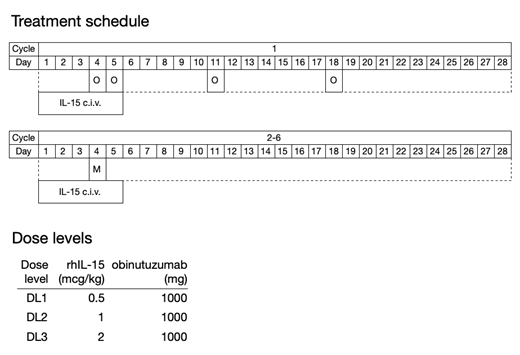
Study design: a single institution non-randomized phase I dose escalation study evaluating increasing doses of civ rhIL-15 in combination with obinutuzumab using a 3 + 3 dose escalation design. On days 1-5 of each 4-week cycle, rhIL-15 will be administered by civ at dose levels 0.5, 1, and 2
mcg/kg/day. During the first cycle, obinutuzumab will be administered at a dose of 100 mg by IV on day 4, 900 mg on day 5, 1,000 mg on day 11, and 1,000 mg on day 18; then 1,000 mg on day 4 of each subsequent cycle. Infusion reaction, antimicrobial, and tumor lysis syndrome prophylaxis will be administered per manufacturer's recommendations. Treatment will continue up to 6 cycles, or until unacceptable toxicity or progressive disease. Up to 24 patients will be enrolled in the study.
Correlative studies: 1) optional lymph node biopsy at baseline and after the first week of treatment (C1D8) for tissue immune cell subsets and quantifying antibody penetration; 2) lymphocyte subset testing, ctDNA, and cell and plasma banking on days 1, 4, 8, and 11 of cycle 1, and days 1 and 8 of cycles 2-6; 3) ADCC capacity of ex vivo NK cells on C1D1, 4, and 8.
One patient has started treatment to date. Enrollment is ongoing.
Wiestner:Acerta: Research Funding; Pharmayclics: Research Funding; Merck: Research Funding; Nurix: Research Funding. Waldmann:Bioniz: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal