Autoimmune cytopenias (AIC) affect 4-7% of patients with chronic lymphocytic leukemia (CLL). Targeted drugs (i.e. ibrutinib, idelalisib and venetoclax) have recently entered the therapeutic armamentarium for CLL showing excellent results in terms of efficacy. The activity of these compounds in CLL-associated AIC is largely unknown, due to the exclusion of patients with active AIC from the pivotal clinical trials and to the paucity of studies directly investigating the role of these novel inhibitors in this setting. Also, no guidelines are available to direct the management of patients who develop AIC during the treatment with targeted drugs.
The purposes of this study were 1) to evaluate the characteristics and outcome of pre-existing AIC in patients with CLL treated with ibrutinib, idelalisib or venetoclax in the real-life setting, and 2) to describe the incidence, quality and management of treatment-emergent AIC during therapy with targeted drugs.
We retrospectively collected data from patients with CLL treated with ibrutinib (n=379), idelalisib (n=100), or venetoclax (n=49) in 11 Italian centers. AIC status was defined as active when it was not controlled with current medical management, controlled/improved when blood counts did not reach the normal values or if subclinical hemolysis was still present, and resolved in the presence of a complete blood count normalization.
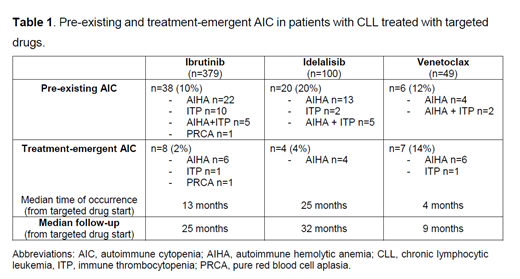
Pre-existing AIC was reported in 38/379 (10%) of ibrutinib-treated patients, 20/100 (20%) of idelalisib-treated patients and 6/49 (12%) of patients who received venetoclax (Table 1).
At the time of the start of ibrutinib, pre-existing AIC was considered active in 8/38 (21%) patients, controlled in 11/38 (29%) and resolved in 19/38 (50%). Among patients with active AIC, ibrutinib treatment induced AIC improvement in 1 patient and a resolution in 4. In addition, during ibrutinib treatment, in 1 patient active AIC was initially controlled but subsequently relapsed at CLL recurrence, in 1 patient concomitant steroid administration was required to maintain AIC controlled and in 1 patient AIC remained stable without needing additional therapy. Controlled AIC remained stable in 3 patients, improved in 3 and resolved in 5. Among 19 patients with a resolved AIC at the time of the start of ibrutinib, only 1 had an AIC relapse during ibrutinib therapy, which was successfully managed with steroids.
At the time of the start of idelalisib, pre-existing AIC was considered active in 5/20 (25%) patients, controlled in 7/20 (35%) and resolved in 8/20 (40%). During idelalisib treatment, AIC improved in 2 patients with active AIC and in 1 patient with controlled AIC, and resolved in 2 patients with active AIC and in 5 patients with controlled AIC. No recurrence was observed in 7/8 patients who had resolved AIC at the time of the start of idelalisib. Overall, a recurrence or worsening of a pre-existing AIC occurred in 3/20 patients.
In the venetoclax cohort, at treatment initiation, pre-existing AIC was active in 2/6 (33%) patients, controlled in 1/6 (17%) and resolved in 3/6 (50%). Active AIC resolved with venetoclax treatment in 1 patient and improved, albeit with concomitant steroid therapy, in the second. The patient with controlled AIC remained stable but needed additional AIC-directed therapy. AIC recurrence was observed in 1/3 patients with resolved AIC and was successfully treated with steroids.
Regarding treatment-emergent AIC, they occurred in 8/379 (2%) patients during ibrutinib therapy, in 4/100 (4%) during idelalisib and in 7/49 (14%) during venetoclax (Table 1). Treatment-emergent AIC significantly correlated with pre-existing AIC in the three cohorts (p<0.05). Interestingly, the percentage of patients with pre-existing AIC who experienced an autoimmune flare during treatment was 10% for ibrutinib and 20% for idelalisib, whereas it was 50% in the venetoclax cohort. Sixty-eight % of patients experiencing treatment-emergent AIC were able to continue the targeted drug at full dose, in combination with additional medications, mainly steroids.
In conclusion, this study based on a large, multicenter, retrospective real-life analysis shows that 1) ibrutinib, idelalisib and venetoclax can induce an improvement or even a resolution of pre-existing AIC in CLL patients, and that 2) treatment-emergent AIC during targeted drugs administration is a rare event, which in most patients is manageable without requiring treatment interruption.
Trentin:ABBVIE: Honoraria, Other: board; Janssen: Consultancy, Honoraria, Other: Board; gilead: Consultancy; Roche: Honoraria, Other: Board. Tedeschi:Janssen spa: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; SUNESIS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BeiGene: Honoraria; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Mauro:Roche: Consultancy, Research Funding; Jannsen: Consultancy, Research Funding; Shire: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding. Foà:Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees; Shire: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Shire: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Boccadoro:Sanofi: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; AbbVie: Honoraria; Mundipharma: Research Funding. Coscia:Karyopharm Therapeutics: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal