Background: Venetoclax (Ven), an orally bioavailable BCL2 inhibitor, is approved as monotherapy (Ven-mono) and in combination with rituximab (R; VenR) for relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and combined with obinutuzumab in 1L CLL. Herein, we present the long-term efficacy data, including durability of response of continuous and fixed-duration therapy, from the initial phase 1b study (Seymour, et al. Lancet Oncol. 2017) of VenR in R/R CLL with median follow-up on study of 4.9 yrs.
Methods: Pts with relapsed CLL received Ven daily (200 - 600 mg) and 6 - 9 doses of R over 6 mo, then Ven-mono (NCT01682616). Minimal residual disease (MRD) was assessed in bone marrow (BM) using multicolor flow cytometry (<10-4 cutoff for undetectable MRD [uMRD]). According to an early revision of the protocol, pts who achieved complete remission (CR) or uMRD regardless of CR status (after completion of VenR) could elect to stop Ven and remain on study, with retreatment at progression.
Results: As of June 4, 2019, the median time on study for the 49 enrolled pts (median 2 prior therapies [range: 1 - 5]) was 4.9 yrs (0 - 6.6); median time on Ven was 2.5 yrs (0 - 6.5). The overall response rate (N=49) was 86%; CR rate: 53%. The 60-mo estimates for overall survival and progression-free survival (PFS) for all were 89% (95% CI: 76 - 95) and 56% (95% CI: 40 - 70), respectively. Among all responders (n = 42), the 60-mo estimate for duration of response (DOR) was 58% (95% CI: 40 - 73). Grade 3 or 4 adverse events (AEs; ≥10%) in pts who received ≥2 yr of continuous treatment were neutropenia (19%) and leukopenia (14%). Serious AEs occurring in >1 pt were pneumonia (n=2) and osteoarthritis (n=2).
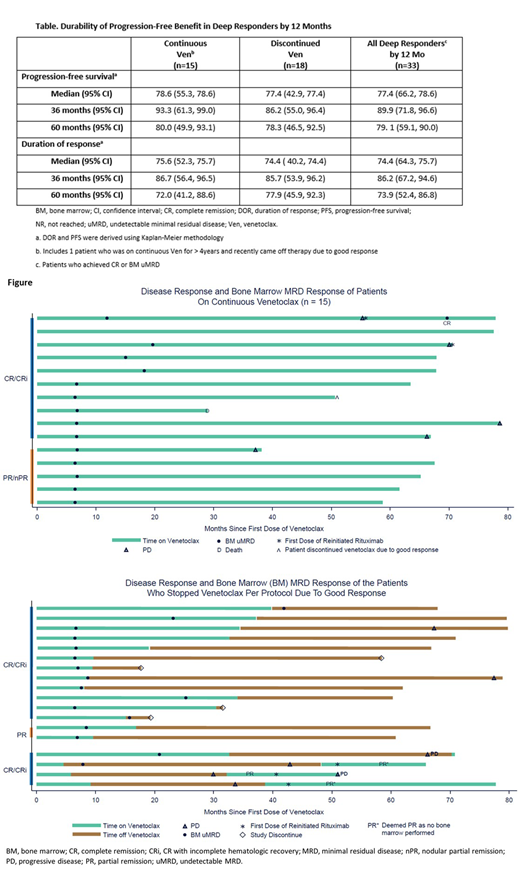
Thirty-three pts (67%) achieved CR or BM uMRD by 12 mo and opted to remain on Ven-mono (n=15) or stopped therapy with Ven due to good response (n=18) and stayed on study; DOR and PFS estimates using Kaplan-Meier methodology are presented in the Table. Of the 16 pts not achieving CR or uMRD by 12 mo and continuing therapy, all subsequently discontinued the trial (withdrew consent [n = 2], AEs [n = 2], progressive disease [PD] with CLL [n = 7], or Richter transformation [n = 5]). One pt (1/16) had PD at 36.9 mo (best response on therapy was partial response [PR]) and received an additional round of R treatment starting at 51.9 mo, but the disease did not respond to intensified treatment and the pt came off Ven at 58.4 mo.
Of the 15 pts (CR or BM uMRD by 12 mo) who remained on Ven-mono long-term, PD occurred in 5 pts and 1 pt died in ongoing response due to myocardial ischemia (unrelated) (Figure). Two pts received an additional round of R treatment after PD, one at 55.3 mo (achieved uMRD CR [DOR post-R, 11.3+ mo]) and the other at 70.5 mo (recently restarted R). At data cut-off, 10 pts were in remission: 6 in CR (including 1 pt retreated with R and 1 pt who recently stopped Ven after 4.3 yr due to being disease-free) and 4 in uMRD PR. Response for the last pt post-R retreatment is pending.
Of the 18 pts who stopped Ven in deep response (14 uMRD CR, 2 MRD-positive CR, 2 uMRD PR [Figure]), the median time on Ven prior to cessation was 16 mo (5 - 40) and median time off Ven is 40.3 mo (1.1 - 70.0) to date. Four have discontinued study without progression (withdrew consent [n = 2], elected for stem cell transplant [n = 1], lost to follow-up [n = 1]). Four pts (2 MRD-positive CR and 2 uMRD CR) had PD after stopping Ven (all asymptomatic) at 25.5, 29.0, 33.3 and 42.0 mo off Ven and have been re-treated with VenR or Ven-mono, and 3 have been re-evaluated for response. All achieved at least PR (2 without BM assessment so unevaluable for CR), with 2 having ongoing response. One pt with PR but residual BM infiltrate had subsequent PD 18 mo later. The fourth pt was recently re-treated with Ven-mono; response is pending. Two additional pts had PD off Ven and had not been re-treated as of the data cutoff; both remain on study.
Conclusions: In relapsed CLL, VenR induces deep responses within 12 mo in 67% of pts. These responses are highly durable whether on continuous or limited duration therapy, with treatment-free remissions of >4 yr now being observed. Re-treatment of pts with Ven or VenR re-exposure has resulted in response in some pts. In addition to long PFS, which represents time to first PD or death, pts who cease Ven in deep response have the opportunity for further disease control through reintroduction of Ven.
Brander:AstraZeneca: Consultancy, Research Funding; Novartis: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy; AbbVie: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Teva: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria, Research Funding; MEI: Research Funding; Acerta: Research Funding; Tolero: Research Funding; BeiGene: Research Funding; DTRM Biopharma: Research Funding. Seymour:Acerta: Consultancy; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding; Roche: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy. Kipps:Genentech, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Velos-Bio: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jannsen Pharmaceutical Companies of Johnson & Johnson: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca, Inc.: Membership on an entity's Board of Directors or advisory committees; Verastem: Membership on an entity's Board of Directors or advisory committees. Ma:Abbvie: Research Funding; Beigene: Research Funding; Janssen: Consultancy, Speakers Bureau; Astra Zeneca: Consultancy, Research Funding, Speakers Bureau; Genentech: Consultancy; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; Acerta: Research Funding; Kite: Consultancy; Bioverativ: Consultancy; Incyte: Research Funding; Juno: Research Funding; Gilead: Research Funding; Novartis: Research Funding; Xeme: Research Funding. Anderson:Walter and Eliza Hall Institute: Employment, Patents & Royalties: Institute receives royalties for venetoclax, and I receive a fraction of these.. Choi:Oncternal: Research Funding; Gilead: Consultancy, Speakers Bureau; Rigel: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau. Humphrey:F. Hoffmann-La Roche Ltd: Employment. Masud:AbbVie: Employment, Other: Stock/stock options. Nandam:AbbVie: Employment, Other: Stock/stock options. Jacobson:AbbVie: Employment, Other: Stock or options. Roberts:Australasian Leukaemia and Lymphoma Group: Membership on an entity's Board of Directors or advisory committees; Walter and Eliza Hall Institute: Patents & Royalties: Institute receives royalties for venetoclax, and I receive a fraction of these.; AbbVie: Other: Unremunerated speaker for AbbVie, Research Funding; Janssen: Research Funding; BeiGene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal