Introduction: Myelodysplastic syndromes (MDS) are hematopoietic stem cell disorders with a tendency to transform to acute myeloid leukemia. Several prognostic systems based almost entirely on clinicopathological characteristics have been developed. However, considering large heterogeneity of MDS, there is an ongoing substantial effort to identify new molecular markers, which would improve patient stratification.
It has recently been discovered that various types of small noncoding RNAs (sncRNAs) are exported into blood circulation. Encapsulated in extracellular vesicles (EVs) or as a part of various molecular complexes, they play important roles in long distance cell-to-cell communication. Their secretion appears to be controlled and specific and thus may reflect (patho)physiological processes occurring in different cells. As diagnostic procedures move from bone marrow biopsies towards less invasive techniques, circulating sncRNAs have become of particular interest as potential novel semi-invasive biomarkers.
Aims: We investigated circulating sncRNAs in MDS on the genome-wide level to better understand MDS pathogenesis and to search specific RNAs applicable as potential biomarkers. We analyzed paired samples from the whole plasma and EV fractions to define which of these two materials is better source of relevant sncRNA-based markers of MDS.
Methods: We performed small RNA-seq in paired samples (plasma and EVs) in 42 patients and 17 healthy controls. To associate sncRNA levels with mutational status, Illumina TruSight Myeloid Sequencing Panel Kit examining 54 genes was applied. Overall survival (OS)‐associated sncRNAs were identified by performing univariate Cox regression along with a permutation test using BRB‐ArrayTools. Multivariate Cox regression analysis with backward variable selection was done to identify independent variables associated with patient survival.
Results: Data analysis showed that the most abundant category of RNAs were miRNAs (51 %), followed by rRNAs, piRNAs, tRNAs, and mRNAs. Sample clustering revealed that RNA content of EV cargo is more homogenous than of plasma. Differential analysis identified a striking disbalance in RNA content between plasma and EVs in MDS patients (> 400 significantly deregulated sncRNAs) whereas these sncRNA profiles remained similar in controls. Such disproportion may suggest that mechanisms of sncRNA export into blood circulation may be specifically affected in MDS. As a result, these changes might substantially affect cell-to-cell communication of blood cells, contributing to the disease development.
Further, we analyzed levels of individual sncRNAs and found that many hematopoiesis-related miRNAs were significantly increased in MDS patients compared to healthy controls, mostly both in plasma and EVs (e.g., miR-34a-5p, miR-125a-5p, and miR-150). Several miRNAs (e.g., miR-103b, miR-107, and miR-221) showed different levels between early and advanced MDS. Interestingly, the majority of miRNAs from the 14q32 miRNA cluster were specifically increased in early MDS.
Additionally, we investigated possible impacts of somatic mutations on expression of circulating sncRNAs. However, only low numbers of circulating sncRNAs significantly associated with mutational status, suggesting no fundamental effects of somatic mutations on sncRNA export from MDS cells.
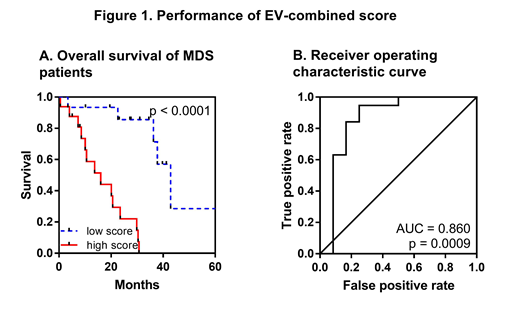
Survival analysis identified sncRNAs with the highest level of association with OS (plasma: miR-1260b, miR-3191-3p, and miR-328-3p; EVs: miR-1237-3p, U33, hsa_piR_019420, and miR-548av-5p). To better stratify MDS patients, we defined risk prediction scores (separately for plasma and EVs) that combined effects of the above mentioned sncRNAs. The results showed that EV-combined score significantly increased the predictive power of the survival risk model (Figure 1). Moreover, Cox multivariate analysis revealed that this score is an independent variable the most significantly associated with OS (HR = 2.942, 95% CI 1.786 to 4.849, p < 0.001).
Conclusions: Our data demonstrate that profile of circulating sncRNAs is specific in MDS and changes with the disease progression. Monitoring of sncRNA levels in EVs provides better prognostic performance for patient survival (compared to whole plasma), probably due to higher homogeneity of the EV fraction.
Supported by grants 16-33617A and 00023736 from the Ministry of Health of the Czech Republic.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal