Decitabine is one of the classical demethylation drugs in the treatment of myelodysplastic syndrome (MDS); however, the exact mechanism of decitabine has not been fully understood. Such knowledge is essential to develop mechanism-based, targeted approaches in the treatment of MDS. Here, we show that decitabine-induced ROS raise leads to ferroptosis in myelodysplastic syndrome cells.
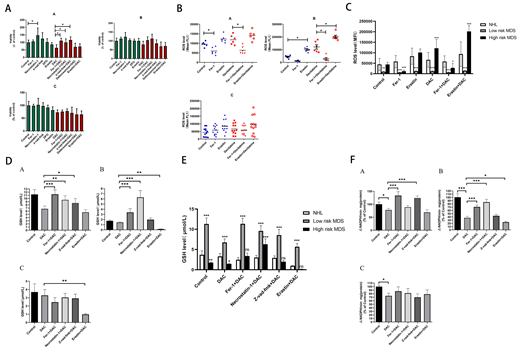
To investigate whether decitabine could induce ferroptosis in MDS cells and its mechanism, cell lines SKM-1 and MUTZ-1 were co-cultured with decitabine and ferroptosis inhibitor (ferrostatin-1), respectively. CCK-8 assay was used to detect the effects of drugs on cell viability. At the same time, we observed whether necroptosis inhibitor (necrostatin-1), apoptosis inhibitor (z-vad-fmk) and iron chelating agent (DFO) could reverse the inhibitory effect of decitabine on MDS cells. The results showed that, necrostatin-1 could increase the cell viability significantly. The growth-inhibitory effect of decitabine on SKM-1 and MUTZ-1 could be partially reversed by ferrostatin-1, DFO and necrostatin-1. The effect of ferrostatin-1 is the most significant. Ferroptosis inducer (erastin) could increase the cytotoxicity of decitabine at different concentrations. Flow cytometry was used to detect the ROS level. Biochemical method was used to detect the intracellular glutathione (GSH) level and glutathione peroxidase (GPXs) activity. The results showed that, the level of GSH and the activity of GPXs decreased while the ROS level increased in SKM-1 and MUTZ-1 cell lines when treated with decitabine, which could all be inhibited by ferrostatin-1.
The iron overload model of C57BL/6 mice was next constructed to observe whether iron overload could induce ferroptosis. The results showed that, the concentration of hemoglobin in peripheral blood of mice was negatively correlated with intracellular Fe2+level and ferritin concentration. Iron overload led to decreased viability of bone marrow mononuclear cells (BMMNCs), which was negatively correlated with intracellular Fe2+level. Ferrostatin-1 and necrostatin-1 partially reversed the decline of cell viability in iron overload groups, and erastin promoted the proliferation of BMMNCs in iron overload mice. The level of GSH and the activity of GPXs decreased while the ROS level increased in BMMNCs of iron overload mice compared with the control. DFO could increase the level of GSH in iron overload mice. Ferrostatin-1, z-vad-fmk and DFO could increase the GPXs activity of BMMNCs in iron overload mice.
Finally, to explore the role of ferroptosis in the pathogenesis of low-risk and high-risk MDS patients respectively, the BMMNCs were obtained from low-risk MDS, high-risk MDS and lymphoma patients respectively and co-cultured with decitabine and above-mentioned inhibitors. The results showed that, ferrostatin-1, necrostatin-1, z-vad-fmk could significantly reverse the inhibitory effect of decitabine of low-risk MDS patients. Necrostatin-1 and Fer-1 could also reverse the inhibitory effect of decitabine of high-risk MDS patients, although the difference was not significant. Decitabine could significantly increase the ROS level in both MDS groups, which could both be inhibited by ferrostatin-1 or promoted by erastin. Ferrostatin-1, necrostatin-1 and z-vad-fmk could significantly reverse the inhibitory effect of decitabine on GSH level in low-risk MDS patients. Ferrostatin-1 and necrostatin-1 could significantly reverse the inhibitory effect of decitabine on GSH level in high-risk MDS patients. Erastin combined with decitabine could further reduce the GSH level, and the difference was significant in high-risk MDS group. For low-risk MDS group, GPXs activity of ferrostatin-1 combined with decitabine and z-vad-fmk combined with decitabine groups were significantly higher than that of decitabine group. For high-risk MDS group, the activity of GPXs of ferrostatin-1 combined with decitabine and necrostatin-1 combined with decitabine groups were significantly higher than that of decitabine group. Erastin could further decrease the activity of GPXs when compared with decitabine group.
Our findings reveal a novel therapeutic mechanism of decitabine and may open a new window for therapeutic targeting in the treatment of MDS.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal