To investigate the clinical impact of U2AF1MT in MDS, we studied a cohort of 511 patients who were performed 112-targeted gene sequencing. We identified U2AF1MT in 86 (17%) subjects, including U2AF1S34F (64%, n=55), U2AF1S34Y (23%, n=20), U2AF1Q157R (6%, n=5), U2AF1Q157P (5%, n=4) and U2AF1R156H (2%, n=2). Compared to many MDS-related gene mutation, U2AF1MT more frequently occurred in younger patients (Median age: 45 vs. 54 yrs; P=0.001). We performed multivariate analysis to characterize the associations between U2AF1MT and other common gene mutations and cytogenetic abnormalities, SF3B1MT (P=0.001, OR=0.214, 95%CI: 0.083-0.55) was inversely associated with U2AF1MT subjects, while isolated +8 (P<0.001, OR=4.671, 95%CI: 2.510-8.689), KRASMT (P=0.008, OR=3.521, 95%CI: 1.388-8.934), and ASXL1MT (P=0.033, OR=2.003, 95%CI: 1.059-3.786) was significantly enriched in U2AF1MT subjects. Using copy number-adjusted VAF, we reconstructed the clonal architecture of U2AF1MT patients to establish whether a U2AF1MT was an ancestral or subclonal mutation. 65 patients with 1 or more mutation, except for U2AF1MT, were analyzed. U2AF1MT was an ancestral mutation in 46 (71%) patients and was a subclonal mutation in 19 (29%) patients. In our cohort, U2AF1MT was associated with worse overall survival (OS, P=0.01). Considering for different type of U2AF1MT, MDS patients with U2AF1Q157/R156MT tended to have a reduced median survival as opposed to patients with S34 (17 vs. 30 months, P=0.318).
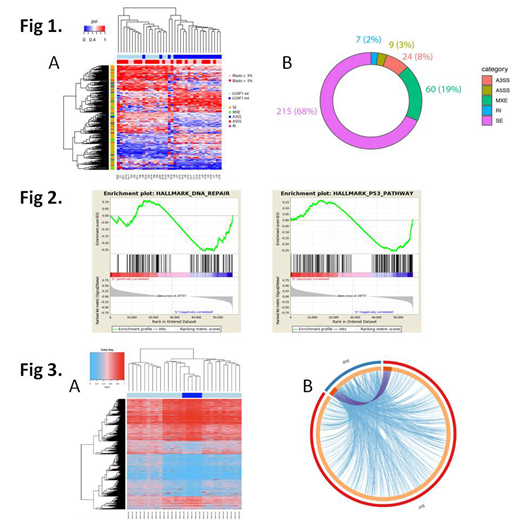
Then, we performed a comprehensive and systematic analysis to determine the impact of U2AF1MT on pre-mRNA splicing in bone marrow mononuclear cells from 40 patients and 5 healthy controls. We identified 315 misregulated splicing events between patients with U2AF1MT (n=20) and those without any spliceosome mutations (n=17) (Figure 1A). U2AF1MT were associated with a higher proportion of exon skipping (SE, 68%), mutually exclusive exons (MXE, 19%) and alternative 3′ splice site (A3SS, 8%) usage events (Figure 1B). Interestingly, only one gene (MRS2, Magnesium Transporter MRS2) with significantly dysregulated expression was observed from the aberrantly spliced genes. Gene ontology (GO) analysis was performed on the genes showing significant aberrant splicing events and the analysis showed a strong cluster of GOs associated with histone modification, RNA metabolism and cell cycle processing. Gene set enrichment analysis (GSEA) revealed DNA damage response pathway, including DNA Repair and p53 pathway as one of the major gene sets up regulated upon U2AF1MT (Figure 2). Then, the single cell RNA-sequencing was performed in Lin-CD34+CD38-CD90+CD45RA- hematopoietic stem cell (HSC) from one sample with U2AF1MT. After QC filtering, 5 HSC with U2AF1MT and 47 HSC without any spliceosome mutations were used for aberrant splicing events analysis. Totally, 1752 misregulated splicing events were indentified (Figure 3A). Forty three genes with significantly dysregulated expression were observed from the aberrantly spliced genes, including 6 genes (TP53BP1, MDM2, PRC1, RAD51C, IP6K2, TMBIM6) taking part in DNA damage response (Figure 3B).
In summary, this comprehensive study provides novel insights into U2AF1MT MDS disease pathophysiology, with newly identified clinical associations, and dysregulated genes and pathways representing potential new therapeutic targets.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal