Background: Myeloproliferative neoplasms (MPNs) are clonal hematopoietic disorders characterized by overproduction of differentiated hematopoietic cells. Among MPNs, chronic myeloid leukemia (CML) with BCR-ABL1 is the best studied subtype and is sometimes diagnosed during childhood. "Ph-negative" MPNs are typically found in older adults and are exceedingly rare during childhood. These classical Ph-negative MPNs include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). Because of this rarity, clinical features of pediatric MPNs are largely unknown, except for CML.
Method: We administered questionnaires to institutions registered with the Japanese Society of Pediatric Hematology/Oncology (JSPHO). We collected clinical information of pediatric and young adult patients aged younger than 30 years who had been diagnosed with Ph-negative MPNs during 2000-2016. This study was conducted as a project of the Leukemia and Lymphoma Committee of the JSPHO.
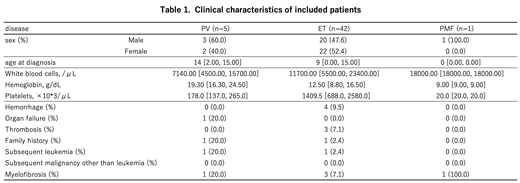
Results: We received responses from 145 institutions, which included all 107 board-certificated educational institutions of the JSPHO. A summary of the clinical features of included patients is shown in Table 1. From 2000 to 2016, 5 patients with PV, 42 with ET, and 2 with PMF were newly diagnosed with Ph-negative MPNs. Among them, 1 patient with PMF was excluded from subsequent analysis because of insufficient clinical information. The male-female ratio was almost balanced. The median age at diagnosis was 14 years for patients with PV, 9 years for patients with ET, and 1 patient with PMF was 10 months old. Only 1 patient with PV had a karyotype abnormality. The median follow-up period was 51 months since diagnosis.
The JAK2V617F mutation was screened in almost all patients, and no patients with PV and 9 patients with ET had this mutation. With regard to treatment, 3 of 5 patients with PV received phlebotomy. Among patients with ET, aspirin was used most frequently (21 patients), followed by anagrelide (11 patients). However, 2 patients with PV and 8 patients with ET received no therapeutic approach. With regard to adverse events, hemorrhage and thrombosis were observed in 4 and 3 patients with ET, respectively, but these events were not found in patients with PV or PMF. Transformation to leukemia or myelofibrosis was found in 1 and 1 patient with PV, and 1 and 3 patients with ET, respectively. Among the patients who received no therapeutic approach, only 1 patient with PV experienced a hemorrhagic event and no other patients experienced any adverse events. Only 1 child with PV died owing to subsequent development of myelofibrosis and acute myeloid leukemia approximately 11 years after the initial diagnosis of PV.
Conclusion: To the best of our knowledge, this is the first nationally-representative survey to clarify the clinical aspects of pediatric patients with Ph-negative MPNs. Although the methodology was not standardized, the frequency of the JAK2V617F mutation appears to be lower than that previously reported for adult patients. Moreover, the incidence of adverse events, such as thrombosis, hemorrhage, leukemia development, and transformation to myelofibrosis, was much lower than that of adult patients. As a result, although at relatively short durations of follow-up, the prognosis of pediatric patients with Ph-negative MPNs appears to be comparatively good. Further study with genetic analysis is warranted to determine the clinical and genetic features of pediatric patients with Ph-negative MPNs.
Mitsui:Astellas pharmaceutica: Research Funding; Daiichi Sankyo pharmaceutical: Research Funding; Chugai pharmaceutical: Research Funding; Teijin pharmaceutical: Research Funding; JCR pharmaceutical: Research Funding; Maruho pharmaceutical: Research Funding; MSD pharmaceutical: Research Funding; Shionogi pharmaceutical: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal