BACKGROUND: The overall survival of patients with newly diagnosed chronic myeloid leukemia (CML) has improved significantly since the introduction of tyrosine kinase inhibitors (TKIs), and recently, some patients with CML who achieve stable deep molecular response (DMR) to TKI therapy could safely suspend treatment. In addition to European LeukemiaNet criteria (ELN), the European Society for Medical Oncology (ESMO) proposed that less than 0.01% of BCR-ABL1 transcript (MR4.0) on the International Scale (IS) after 18 months is optimal for patients to achieve treatment-free remission. Branford et al. indicated that major molecular response (MMR; less than or equal to 0.1% of BCR-ABL1 IS ; MR3.0) at three months predicts stable undetectable BCR-ABL1 transcript levels during imatinib treatment. However, second-generation TKIs induce more rapid and deeper responses at early time points compared to imatinib; however, whether early molecular response predicts MR4.0 by 18 months to second-generation TKI therapy, remains unclear.
AIMS: We retrospectively analyzed BCR-ABL1 transcript levels in patients with CML in chronic phase (CML-CP) at 3, 6, 12, and 18 months after initiating dasatinib treatment to identify molecular milestones that would predict MR4.0 by 18 months.
METHODS: Fifty-nine newly diagnosed patients with CML-CP were included in this study. The median age was 61 years (18-81 years), 72% of whom were males, and patients older than 65 years comprised 31% of the overall enrollment. Eighty-six percent of them had low and intermediate Sokal scores. Patients received 100 mg of dasatinib once daily. Dose interruption or reduction was allowed if drug-related grade 3 non-hematological toxicity or grade 3 or worse hematological toxicity occurred.BCR-ABL1/ ABL ratio IS (BCR-ABL1 IS) was performed at approximately 3-month intervals. To assess the kinetics of response, we calculated halving time (HT) of BCR-ABL1 IS. HT-BCR-ABL1 was calculated as described by Branford et al. We used a receiver-operating characteristic (ROC) curve to identify the cut-offs in transcript levels at three and six months. Mann-Whitney test was used to determine statistical significance. Categorical variables were compared using Fisher's exact test. Factors were subjected to multivariate analysis using Cox regression; differences with p<0.05 were considered statistically significant.
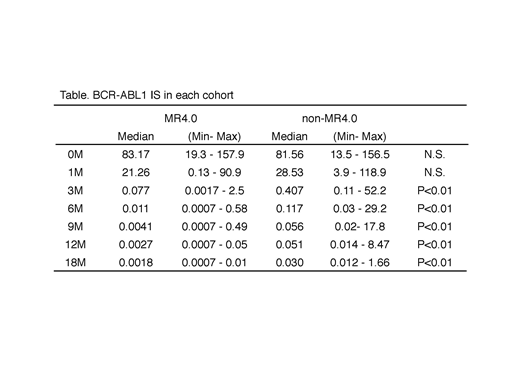
RESULTS: The median BCR-ABL1 IS level before therapy was 82.1% (range 13.5-157.9 %). The estimated MMR by 12 months and MR4.0 by 18 months were 86.4% and 50.8%, respectively. We analyzed each into a cohort that achieved MR4.0 by 18 months (MR4.0 cohort) and a cohort that did not achieve MR4.0 (non-MR4.0 cohort). The BCR-ABL1 IS level in each cohort is indicated in the Table. Median HT-BCR-ABL1 IS 0-3 months was 10.8 days (6.5-71.3 days) in MR4.0 cohort, and 13.0 days (8.5- 169.6 days, p<0.05) in non-MR4.0 cohort, whereas that of 3-6 months was 24.4 days (6.5-228.9 days) in MR4.0 cohort and 47.3 days (18.0- 154.9 days, p<0.01) in non-MR4.0 cohort. The shape of BCR-ABL1 IS descent was biphasic in MR4.0 cohort. The cut-off values of BCR-ABL1 IS at 3 months and 6 months for prediction of MR4.0 by 18 months were 0.122% (specificity 0.964, sensitivity 0.586, AUC 0.784) and 0.048% (0.852, 0.833, 0.909), respectively. We determined the optimal threshold for predicting MR4.0 by 18 months using these parameters. Some of them (BCR-ABL1 IS 3 months ≤0.122%,BCR-ABL1 IS 6 months ≤ 0.048%, BCR-ABL1 IS 6 months ≤1%: ELN optimal criteria) were identified as predictive cut-off values for MR4.0 by 18 months in univariate analysis (BCR-ABL1 IS 3 months ≤0.122%; 94.4% vs. 30.7%, p<0.01, BCR-ABL1 IS 6 months ≤ 0.048%; 85.7% VS. 20.7%, p<0.01 and BCR-ABL1 IS 6 months <1.0%; 58.8% vs. 0%, p<0.01). However, BCR-ABL1 IS 3 months ≤ 10% (ELN optimal criteria at three months) was not identified as predictive cut-off value for MR4.0 (54.7% vs. 20%, N.S.). Multivariate analysis indicated that BCR-ABL1 IS 6 months ≤ 0.048% was a significant independent factor for achievement of MR4.0 by 18 months (hazard ratio 0.05, p<0.01).
CONCLUSION: Even though dasatinib is highly-priced, dasatinib treatment results in higher rates of molecular responses in newly diagnosed patients with CML-CP. Assessment of molecular reduction within six months has become an important landmark to predict MR4.0 by 18 months during dasatinib treatment for patients with the aim to achieve treatment-free remission.
Oyake:Astellas: Speakers Bureau; Celgene: Honoraria; Chugai: Honoraria; Kyowa-Hakko Kirin: Honoraria. Ito:Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Ono: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal