Background: We have previously shown that the survival of patients with AML who fail to achieve complete remission (CR) with 7+3 has improved since the 1980s. However, although CR rates with 7+3 have improved over the last four decades, we have not previously evaluated how outcomes for patients who achieve a CR1 with 7+3 has changed over time. Here we evaluate if either length of first CR (CR1) after 7+3 or of survival after relapse from CR1 has changed over the last four decades.
Patients and Methods:We analyzed 1247 patients randomized to 7+3 arms from 5 SWOG studies and restricted to patients age 65 or younger: S8600 (n=530), S9031 (n=98), S9333 (n=57), S0106 (n=301), S1203 (n=261). S8600 enrolled patients in the 1980s, S9031 and S9333 in the 1990s, S0106 in the 2000s, and S1203 in the 2010s. S9031 and S9333 were analyzed together. All 5 protocols gave 7+3 per existing standard, which changed over time. In S8600, S9031, and S9033 the ara-C and daunorubicin doses were 200mg/m2and 45mg/m2, in S0106 100mg/m2and 60mg/m2, and in S1203 200mg/m2and 90mg/m2. CR was defined morphologically. To account for censoring in the dataset, we used landmark analyses. To evaluate patterns in length of CR1, among patients achieving CR1 and alive at 2 and 3 years, we calculated the proportion of 2 (or 3) years spent in CR1. To evaluate survival after relapse, among patients who achieved CR1 but who relapsed in next 2 (or 3) years we calculated the proportion of patients alive at least 1 year after relapse. To account for changing patient characteristics over time, multivariate linear and logistic regression models were fit.
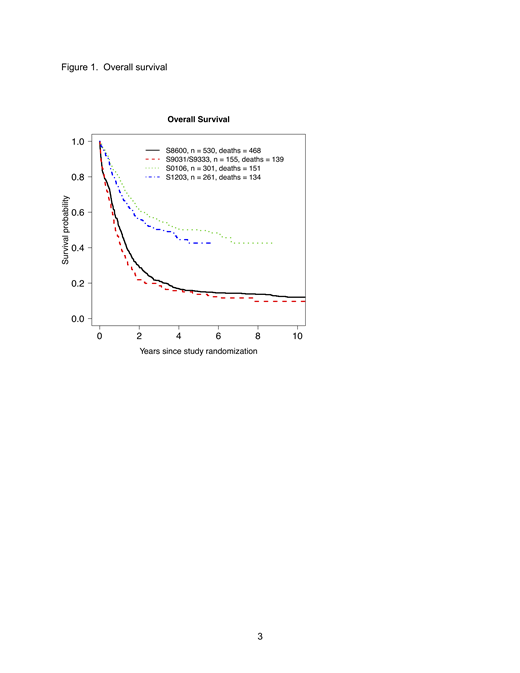
Results:Overall survival has improved dramatically over the last 4 decades (Figure 1). Additionally, among patients who achieved CR1 and were alive 2 years later, the proportion of those 2 years spent in CR1 has significantly improved over the last 4 decades (Figure 2) from a median of 58% to a median of 96% (p<0.001). This trend was maintained in multivariable modeling (Table 1) and similar trends were observed using a 3-year landmark time. Survival after relapse also improved over the 4 decades. Among patients who relapsed in the first 2 years after CR1, the proportion alive at least one year after relapse has significantly increased from 32% to 52% (Table 2, p<0.0001). This pattern was maintained in multivariable modeling adjusting for patient prognostic factors (Table 3) and similar trends were observed using a 3-year landmark time.
Conclusion:Both length of CR1 and survival after relapse have increased over the last four decades in patients age 65 or younger even after accounting for differences in patient characteristics. Possible explanations for the longer survival after relapse include higher 2ndCR rates, more frequent use of hematopoietic cell transplant in CR, or better supportive care. Regardless, the longer survival after relapse suggests analyses of event-free survival should complement those of overall survival when evaluating new treatments in AML.
Acknowledgement:The authors wish to gratefully acknowledge the important contributions of the late Dr. Stephen H. Petersdorf to SWOG and to study S0106.
Othus:Glycomimetics: Other: Data Safety and Monitoring Committee; Celgene: Other: Data Safety and Monitoring Committee. Garcia-Manero:Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding; Amphivena: Consultancy, Research Funding. Erba:Agios, Amgen, Astellas Pharma, Daiichi Sankyo, ImmunoGen, Janssen, Jazz Pharmaceuticals, Juno, Millennium, Seattle Genetics: Research Funding; Amgen, Celgene, Daiichi Sankyo, ImmunoGen, Incyte, Jazz Pharmaceuticals, Millennium, Novartis, Ono, Pfizer, Seattle Genetics, Sunesis: Consultancy; Celgene, Incyte, Novartis: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal