Introduction: Up to 40% of patients (pts) with previously untreated diffuse large B-cell lymphoma (DLBCL) fail to achieve remission, or relapse, with standard-of-care rituximab (R) plus CHOP (R-CHOP). Atezolizumab (atezo) is a fully humanized anti-programmed death-ligand 1 (PD-L1) antibody with a complementary mode of action to R. We present updated data from an ongoing Phase I/II study (NCT02596971) evaluating the safety and efficacy of atezo combined with R-CHOP (R-CHOP-atezo) in pts with previously untreated DLBCL. This is an updated analysis performed at the end of consolidation (EOC).
Methods: This open-label, multicenter study enrolled adults with previously untreated advanced DLBCL (ECOG performance status 0-2). Pts received induction treatment with R-CHOP-atezo (8 x 21-day cycles of R [375mg/m2 i.v. on Day 1 (Cycles 1-8)] and atezo [1200mg i.v. on Day 1 (Cycles 2−8)], and 6 or 8 cycles of CHOP, as determined by the investigator [INV]). Pts who had a complete response (CR) at end of induction (EOI) received consolidation treatment with atezo 1200mg i.v. on Day 1 of Cycles 9─25, every 21 days for 12 months. Pts are followed for 12 months after EOC. Primary endpoints were safety and efficacy as determined by CR rate at EOI by an independent review committee (IRC) using Lugano 2014 criteria (modified to include required confirmation of CR at EOI by biopsy in cases with bone marrow involvement at baseline, and confirmation of a PET-based partial response [PR] by CT-based CR or PR). Secondary endpoints included progression-free survival (PFS) and overall survival (OS).
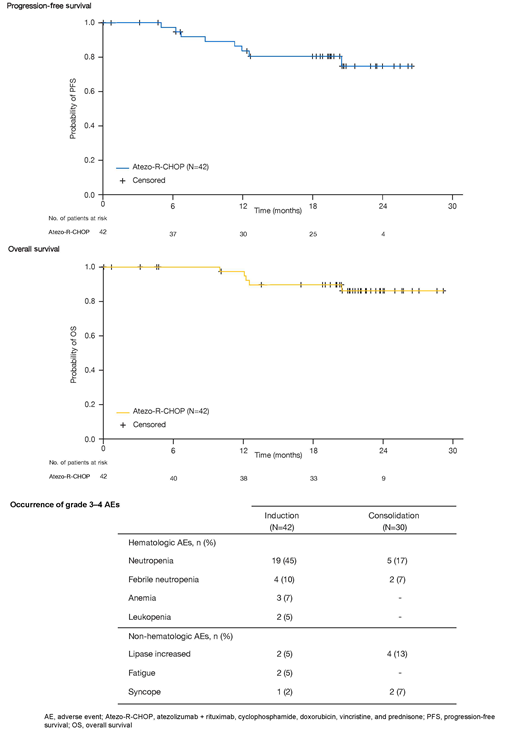
Results: As of May 2019, 42 pts had enrolled and received treatment. Of these, 7 pts discontinued study treatment prior to EOI (protocol violation [n=1], adverse event [AE; n=4], progressive disease [PD; n=1] and withdrawn consent [n=1]); 5 more pts discontinued at EOI (PD [n=2] and PR [n=3]). Of 30 pts initiating consolidation treatment, 15 discontinued (AE [n=9], PD [n=3] and withdrawn consent [n=3]). Median observation time was 21.3 (0.7-29.2) months. Key baseline characteristics included: median age, 65 years; International Prognostic Index (IPI) score ≥3, 69%; cell of origin (COO; Nanostring): ABC (34%), GCB (53%), unclassified (12.5%). Among the 40 pts who received at least one dose of atezo, and were therefore evaluable for efficacy at EOI, 31 (77.5%) had a CR and 4 (10%) had a PR by IRC; PD occurred in 2 (5%) pts; 3 (7.5%) were not evaluable. At 6, 12, 18 (EOC) and 24 months, Kaplan-Meier estimates (95% CI) of INV-assessed PFS were 97.4% (82.8, 99.6), 83.6% (67.0, 92.3), 80.6% (63.5, 90.3) and 74.9% (54.3, 87.2), respectively, and those for OS were 100% (not evaluable), 97.5% (83.6, 99.6), 89.8% (75.1, 96.1) and 86.4% (70.0, 94.2), respectively. Kaplan-Meier survival curves for PFS and OS are shown in the Figure. All 42 pts in the safety population experienced ≥1 AE of any grade, with neutropenia (52.4%), constipation (42.9%) and fatigue (40.5%) the most common. Grade 3-4 AEs occurred in 28/42 (67%) pts during induction and 15/30 (50%) pts during consolidation. Hematologic events were the most common grade 3-4 AEs (Table). One fatal AE (unconfirmed progressive multifocal leukoencephalopathy) was reported during follow-up. Overall, 24% of pts had an AE of special interest (AESI). The most common AESIs during induction were lipase increased (n=1), hyperthyroidism (n=1) and amylase increased (n=1), and those during consolidation were lipase increased (n=2), pancreatitis (n=2) and hepatitis (n=2). These events were generally well managed by atezo discontinuation and steroid treatment, and were largely reversible. AEs led to discontinuation of any treatment in 15 (36%) pts. AEs that led to discontinuation of any treatment in ≥1 pt were neutropenia (n=3), lipase increased (n=3) and amylase increased (n=2). Despite a relatively high number of discontinuations due to AEs during consolidation, events were generally manageable and reversible and all pts maintained response at the time of the analysis. Exploratory biomarker data and minimal residual disease data will be presented.
Conclusions: The EOI PET-CR response rate and preliminary PFS with R-CHOP-atezo are encouraging and at least comparable to those previously reported for R-CHOP. The overall safety profile of R-CHOP-atezo appears manageable and as expected, with no new safety signals reported with consolidation and no overall increase in toxicity other than immune-mediated events.
Younes:BMS: Research Funding; Epizyme: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; HCM: Consultancy; Genentech: Research Funding; Syndax: Research Funding; Xynomics: Consultancy; Biopath: Consultancy; Takeda: Honoraria; Abbvie: Honoraria; Roche: Consultancy, Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Curis: Honoraria, Research Funding; Merck: Honoraria, Research Funding; AstraZeneca: Research Funding; Pharmacyclics: Research Funding. Burke:Celgene: Consultancy; Roche/Genentech: Consultancy; Gilead: Consultancy. Cheson:Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trillium: Research Funding; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Research Funding; Bristol Myers Squibb: Research Funding; Portola: Research Funding; Kite: Research Funding; Gilead: Research Funding; Epizyme: Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Acerta: Consultancy, Research Funding; Symbios: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Diefenbach:Bristol-Myers Squibb: Consultancy, Research Funding; Denovo: Research Funding; Genentech: Consultancy, Research Funding; Incyte: Research Funding; LAM Therapeutics: Research Funding; MEI: Research Funding; Merck: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Millenium/Takeda: Research Funding; Trillium: Research Funding. Hahn:Roche: Other: Roche paid airfare and accommodation for attendance at ASH 3 years ago . Hawkes:Takeda: Speakers Bureau; Merck Sharp & Dohme: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; Astra Zeneca: Research Funding; Roche/Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Speakers Bureau; Bristol-Myers Squibb: Research Funding, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck KgA: Research Funding; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Mundi pharma: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding. Khan:Genentech, Inc.: Honoraria, Research Funding, Speakers Bureau. Lossos:NIH: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Janssen Scientific: Membership on an entity's Board of Directors or advisory committees. Vitolo:F. Hoffmann-La Roche: Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees. Yuen:Seattle Genetics, Inc.: Other: Travel expenses, Research Funding. Raval:Roche: Employment, Equity Ownership. Shivhare:F. Hoffmann-La Roche Ltd: Employment. Nielsen:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Sellam:Roche: Employment, Equity Ownership. Sharman:AstraZeneca: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; TG Therapeutics: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Acerta: Consultancy, Honoraria, Research Funding.
Atezolizumab (atezo) is a programmed death-ligand 1 (PD-L1) blocking antibody. In the United States, atezo is approved for treatment of pts with locally advanced or metastatic urothelial carcinoma who are: not eligible for cisplatin-containing chemotherapy (chemo) and whose tumors express PDL-1, or are not eligible for any platinum-containing chemo regardless of PD L1 status; or have disease progression during or following any platinum-containing chemo, or within 12 months of neoadjuvant or adjuvant chemo. Atezo is also approved: in combination with bevacizumab, paclitaxel and carboplatin for first-line treatment of pts with metastatic non-squamous non-small-cell lung carcinoma (NSCLC) with no EGFR or ALK genomic tumor aberrations, and for pts with metastatic NSCLC who have disease progression during or following platinum-containing chemo; in combination with paclitaxel protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1; and in combination with carboplatin and etoposide, for the first-line treatment of adults with extensive-stage small cell lung cancer. Atezo is not approved for treatment of pts with multiple myeloma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal