Introduction:Programmed death-1 (PD-1) inhibitors are highly active in relapsed and refractory (RR) classical Hodgkin lymphoma (cHL), however their role as part of second-line therapy (SLT) for cHL is largely unexplored. The standard approach following front-line treatment failure is SLT, aimed to achieve complete response (CR), followed by consolidation with high dose therapy and autologous stem cell transplant (HDT/ASCT). There is no one standard SLT and options include platinum-containing regimens, gemcitabine-containing regimens and more recently brentuximab vedotin (BV)-containing regimens. Due to the increasing use of BV in the front-line setting, development of SLT regimens that are both highly effective and BV-sparing are needed. In this phase II study, we aimed to establish the safety and efficacy of SLT with the PD-1 inhibitor, pembrolizumab, combined with the outpatient-administered salvage regimen, GVD (gemcitabine, vinorelbine, liposomal doxorubicin).
Methods: Transplant eligible patients (pts) with RR cHL following failure of 1-line of therapy are eligible. Treatment consists of 2 to 4 cycles of pembrolizumab (200mg IV, day 1), gemcitabine (1000mg/m2 IV, days 1 and 8), vinorelbine (20mg/m2 IV, days 1 and 8) and liposomal doxorubicin (15mg/m2, days 1 and 8), given on 21-day cycles. Response is assessed by PET after 2 and 4 cycles. Pts who achieve CR by PET (Deauville ≤3) after 2 or 4 cycles proceed to HDT/ASCT. An initial safety assessment for the first 6 treated pts was completed before continuing further enrollment. We report here the results of the safety assessment and the first stage of a Simon 2-stage design.
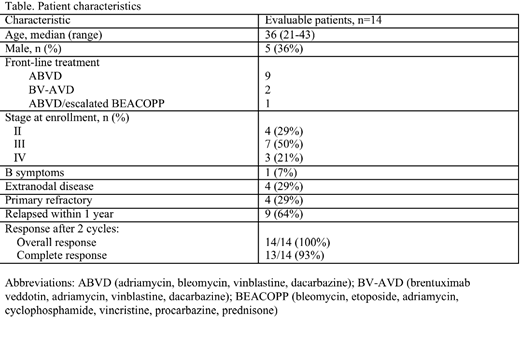
Results:To date, 18 out of a planned 39 pts enrolled; 14 completed the first 2 cycles of treatment and underwent the first response assessment. Characteristics for the 14 evaluable pts are shown in the table. In brief, median age is 36 (range 21-43), 4 (29%) have primary refractory disease and 9 (64%) relapsed within the first year of initial treatment. No dose limiting toxicities were observed during the safety assessment and no significant adverse events were observed to date. Of the 30 cycles administered, 5 (17%) cycles were delayed due to treatment related adverse events which included grade 3 rash (3%) and grade 3 liver function test abnormalities (13%). Among the 14 evaluable pts, 13 (93%) achieved CR after 2 cycles of treatment and 1 achieved partial response. To date, 3 pts are proceeding to HDT/ASCT and 11 pts completed HDT/ASCT following 2 (n=10) or 4 (n=1) cycles of treatment. Median follow-up post HDT/ASCT is 4 mos (range 0.3-8.8 mos) and all pts remain in remission to date.
Conclusion:Early trial results suggest that pembrolizumab-GVD is a highly effective and well-tolerated regimen that can efficiently bridge pts with RR cHL to HDT/ASCT. Efficacy criteria for stage one of the Simon 2-stage design was met and enrollment continues to better characterize CR rate and tolerability.
Moskowitz:ADC Therapeutics: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Erytech Pharma: Consultancy; Takeda Pharmaceuticals: Consultancy; Takeda Pharmaceuticals: Consultancy; miRagen Therapeutics Inc: Consultancy, Research Funding; Incyte: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Cell Medica: Consultancy; miRagen Therapeutics Inc: Consultancy, Research Funding; Cell Medica: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Merck: Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Merck: Research Funding; Cell Medica: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Merck: Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; ADC Therapeutics: Consultancy; Erytech Pharma: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Erytech Pharma: Consultancy; ADC Therapeutics: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Erytech Pharma: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Erytech Pharma: Consultancy; Erytech Pharma: Consultancy; miRagen Therapeutics Inc: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Takeda Pharmaceuticals: Consultancy; Cell Medica: Consultancy; Erytech Pharma: Consultancy; Cell Medica: Consultancy; Cell Medica: Consultancy; Incyte: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Incyte: Research Funding; Incyte: Research Funding; Cell Medica: Consultancy; Incyte: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; Incyte: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Takeda Pharmaceuticals: Consultancy; Merck: Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Erytech Pharma: Consultancy; miRagen Therapeutics Inc: Consultancy, Research Funding; Erytech Pharma: Consultancy; miRagen Therapeutics Inc: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Incyte: Research Funding; Erytech Pharma: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Cell Medica: Consultancy; Merck: Research Funding; Takeda Pharmaceuticals: Consultancy; Merck: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; ADC Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Erytech Pharma: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Merck: Research Funding; Merck: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Erytech Pharma: Consultancy; Erytech Pharma: Consultancy; Incyte: Research Funding; Cell Medica: Consultancy; miRagen Therapeutics Inc: Consultancy, Research Funding; Cell Medica: Consultancy; Incyte: Research Funding; Cell Medica: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; Cell Medica: Consultancy; Takeda Pharmaceuticals: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Takeda Pharmaceuticals: Consultancy; Incyte: Research Funding; Takeda Pharmaceuticals: Consultancy; Takeda Pharmaceuticals: Consultancy; Cell Medica: Consultancy; Cell Medica: Consultancy; Cell Medica: Consultancy; Takeda Pharmaceuticals: Consultancy; Merck: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Incyte: Research Funding; ADC Therapeutics: Consultancy; Incyte: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Incyte: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Merck: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Merck: Research Funding; Erytech Pharma: Consultancy; Incyte: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Merck: Research Funding; Takeda Pharmaceuticals: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Consultancy; Erytech Pharma: Consultancy. Shah:Amgen: Research Funding; Janssen: Research Funding. Kumar:Seattle Genetics: Research Funding. Batlevi:Juno Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Straus:Seattle Genetics: Consultancy, Honoraria; Elsevier (PracticeUpdate): Consultancy, Honoraria; Hope Funds for Cancer Research: Membership on an entity's Board of Directors or advisory committees. Rodriguez-Rivera:Memorial Sloan Kettering Cancer Center: Employment. Colbourn:ABBV: Other: Stock; CELG: Other: Stock; BIIB: Other: Stock; SGEN: Other: Stock; JNJ: Other: Stock; LLY: Other: Stock; GILD: Other: Stock; MRK: Other: Stock; SNY: Other: Stock. Horwitz:Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kura: Consultancy; Celgene: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium/Takeda: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Affimed: Consultancy; Aileron: Research Funding; Trillium: Research Funding; ADCT Therapeutics: Research Funding; Affimed: Consultancy; Astex: Consultancy; Infinity/Verastem: Consultancy, Research Funding; Trillium: Research Funding; Astex: Consultancy; Aileron: Research Funding; Seattle Genetics: Consultancy, Research Funding; Millennium/Takeda: Consultancy, Research Funding; Kyowa Hakko Kirin: Consultancy; Infinity/Verastem: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Forty-Seven: Research Funding; Celgene: Consultancy, Research Funding; ADCT Therapeutics: Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Consultancy, Research Funding; Portola: Consultancy; Forty-Seven: Research Funding; Aileron: Research Funding; Seattle Genetics: Consultancy, Research Funding; Aileron: Research Funding; Forty-Seven: Research Funding; Trillium: Research Funding; ADCT Therapeutics: Research Funding; Astex: Consultancy; Trillium: Research Funding; Forty-Seven: Research Funding; Affimed: Consultancy; ADCT Therapeutics: Research Funding; Affimed: Consultancy; Astex: Consultancy; Innate Pharma: Consultancy; Innate Pharma: Consultancy; Kyowa Hakko Kirin: Consultancy; Mundipharma: Consultancy; Innate Pharma: Consultancy; Infinity/Verastem: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Kura: Consultancy; Kyowa Hakko Kirin: Consultancy; Kura: Consultancy; Kyowa Hakko Kirin: Consultancy; Miragen: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Millennium/Takeda: Consultancy, Research Funding; Mundipharma: Consultancy; Miragen: Consultancy; Miragen: Consultancy; Portola: Consultancy; Mundipharma: Consultancy; Miragen: Consultancy; Kura: Consultancy; Innate Pharma: Consultancy; Mundipharma: Consultancy; Portola: Consultancy; Portola: Consultancy. Palomba:Hemedicus: Other: Immediate Family Member, Speakers Bureau ; Merck & Co Inc.: Other: Immediate Family Member, Consultancy (includes expert testimony); Seres Therapeutics: Other: Immediate Family Member, Equity Ownership and Membership on an entity's Board of Directors or advisory committees; STRAXIMM: Other: Immediate Family Member, Membership on an entity's Board of Directors or advisory committees; Kite Pharmaceuticals: Other: Immediate Family Member, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Noble Insights: Consultancy; Evelo: Other: Immediate family member, Equity Ownership; MSK (IP for Juno and Seres): Other: Immediate Family Member, Patents & Royalties - describe: intellectual property rights . Noy:Prime Oncology: Honoraria; NIH: Research Funding; Janssen: Consultancy; Medscape: Honoraria; Pharamcyclics: Research Funding; Raphael Pharma: Research Funding. Matasar:Genentech, Inc.: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding; Daiichi Sankyo: Consultancy; Bayer: Consultancy, Honoraria, Other; Roche: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding; Merck: Consultancy, Equity Ownership; Juno Therapeutics: Consultancy; Teva: Consultancy; Rocket Medical: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Other: Travel, accomodation, expenses, Research Funding; GlaxoSmithKline: Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Bayer: Other: Travel, accommodation, expenses; Janssen: Honoraria, Research Funding. Vardhana:Rheos Pharmaceuticals: Honoraria; ADC Therapeutics: Consultancy; Agios Pharmaceuticals: Honoraria. von Keudell:Bayer: Consultancy; Pharmacyclics: Consultancy; Pharmacyclics: Consultancy; Genentech: Consultancy; Genentech: Consultancy; Bayer: Consultancy. Younes:Roche: Consultancy, Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Curis: Honoraria, Research Funding; Merck: Honoraria, Research Funding; Abbvie: Honoraria; Takeda: Honoraria; Pharmacyclics: Research Funding; AstraZeneca: Research Funding; Genentech: Research Funding; Biopath: Consultancy; Xynomics: Consultancy; Epizyme: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; HCM: Consultancy; BMS: Research Funding; Syndax: Research Funding. Zelenetz:Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics/AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics/AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Moskowitz:Genentech: Consultancy, Research Funding; Seattle Genetics, Inc.: Consultancy, Research Funding; Celgene: Consultancy; Pharmacyclics: Research Funding; ADC Therapeutics: Research Funding; Merck: Consultancy, Research Funding.
Pembrolizumab is not approved for second-line use for classical Hodgkin lymphoma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal