Background: Adult patients with relapsed B-cell acute lymphoblastic leukemia have a poor prognosis. Central nervous system (CNS) involvement is not often detected at initial diagnosis however, in the absence of CNS-directed prophylactic therapy, CNS relapse will occur in the majority of patients. Our lab has previously discovered a direct route for ALL cells to invade from the bone marrow to the CNS. ALL cells were shown to migrate along emissary vessels between the calvarial or vertebral bone marrow to the subarachnoid space in an α6 integrin-laminin dependent manner. GS-649443, the tool compound of the PI3Kδ inhibitor idelalisib, was shown to decrease integrin α6 expression and inhibit migration to the CNS along these channels without greatly affecting bone marrow disease burden. Here, we investigate the combined PI3Kα/δ inhibitor, copanlisib, as a potentially superior agent in suppressing ALL systemic disease burden and reducing migration to the CNS.
Methods: We tested a panel of four different B-ALL cell lines (Nalm-6, RCH-ACV, SUP-B15 and REH) for in vitro assays, where the PI3K inhibitors and cytarabine were used at concentration of 100 nM. Cell cycle analysis was achieved using Hoechst and Ki67 staining; apoptosis was determined using Annexin V and propidium iodide or 7-AAD staining. SCID mice were engrafted with 5 x 106 ALL cells and administered intravenously with 14 mg/kg copanlisib twice a day once weekly. ALL cells were exposed to cytarabine in vitro for 3 hrs followed by 3 PBS washes prior to copanlisib treatment.
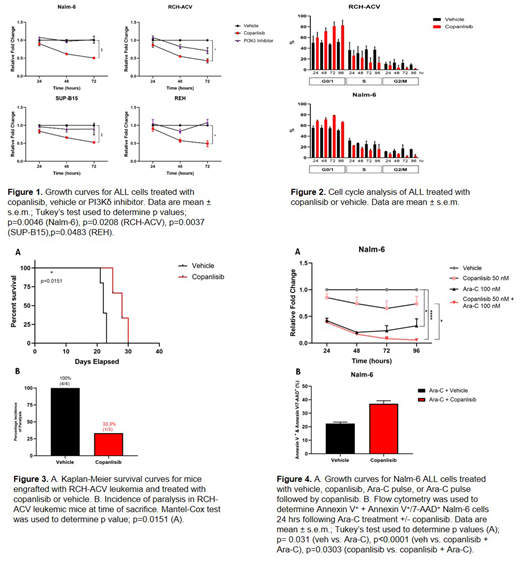
Results: Copanlisib inhibited cell proliferation more effectively than PI3Kδ inhibition in all B-ALL cell lines tested (Figure 1). This is due in part to a G0/1 cell cycle arrest observed following treatment (Figure 2). Copanlisib was also shown to decrease integrin α6 surface expression, laminin adhesion, myosin light chain phosphorylation, and ALL invasion along a laminin enriched matrix. Congruently, in vivo studies showed an increased survival benefit in ALL-engrafted SCID mice, with a lower incidence of paralysis secondary to CNS metastasis in the copanlisib treated arm (Figure 3).
PI3K signaling can be enhanced as part of the cellular stress response, resulting in downstream anti-apoptotic pathway activation that increases survival under toxic conditions. We therefore hypothesized that PI3Kα/δ inhibition could potentiate chemotherapy treatment by preventing the induction of anti-apoptotic signaling responses. We found that ALL cells increased their Akt-dependent PI3K signaling after cytarabine chemotherapy, as shown by increased Akt and GSK3β phosphorylation. When ALL cells were treated with copanlisib after cytarabine exposure, combined therapy resulted in decreased Nalm-6 cell proliferation and increased apoptosis in vitro (Figure 4). Furthermore, leukemic mice showed a survival benefit when administered cytarabine in combination with PI3K inhibition.
Conclusion: Our preclinical research shows that copanlisib is an effacious drug for the treatment of ALL. We demonstrate that copanlisib inhibits ALL proliferation through induction of cell cycle arrest and decreases ALL invasion by blocking integrin-mediated cell migration. We also show that copanlisib treatment after chemotherapy can enhance ALL cell kill by blunting cytarabine-induced anti-apoptotic signaling responses. These observations translate into significant therapeutic benefit in leukemic mice, where copanlisib both increases survival and inhibits ALL metastasis to the CNS.
Sipkins:National Institutes of Health: Research Funding; American Cancer Society: Research Funding; GlycoMimetics, Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal