One strategy to improve precision medicine in acute myeloid leukemia (AML) is to further our understanding of the biological factors that influence pharmacologic efficacy. In a recent study, whole transcriptome analysis was conducted using pre- and post-treatment samples from a cohort of relapsed or refractory (R/R) AML patients treated with mitoxantrone, etoposide, cytarabine (MEC), and ixazomib. Logistic regression and linear discriminant analysis identified RORa as a predictor of response to treatment (Advani, et al., Clin Cancer Res, 2019, 4231-4237). RORa is not frequently mutated in AML but is described to be a tumor suppressor in patients with solid tumors. In the latter patient population, increased RORa expression is also associated with improved survival.
We first retrospectively evaluated the prognostic significance of RORa in a larger cohort of R/R patients with AML (BeatAML). Second, we characterized RORa expression in cellular models of AML and sought to determine whether a commercially available RORa/RORγ agonist, SR1078, has anti-proliferative capacity in leukemia cell lines.
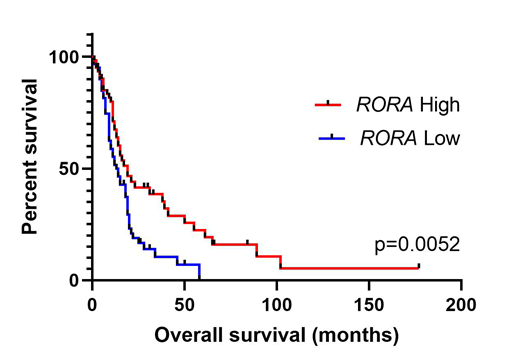
For the correlative survival analyses, RORa mRNA expression and clinical information was downloaded from the BeatAML cohort (Tyner, et al., Nature, 2018, 526-531). Patients were categorized into high expressers (≥ median) and low expressers (<median). In total, 121 R/R patients were investigated. The mean age of diagnosis was 62 ± 12 years, and 58% of patients were male. The most common specific diagnosis at inclusion was AML with myelodysplasia related changes (28%), followed by AML with mutated NPM1 (18%) and therapy-related myeloid dysplasia (13%). Over half of the analyzed samples were peripheral blood (58%), and the remaining samples were either bone marrow aspirate (58%) or from leukapharesis (2%). As a whole, the median mRNA expression levels of RORa in patients with R/R AML (n=121) compared to healthy subjects (n=21) were 6.6 log2 CPM vs. 2.5 log2 CPM (P<.0001). After grouping patients into low RORa expresser and high RORa expresser groups, patients with above median expression of RORa were found to have significantly longer overall survival (19 mo. vs. 13 mo.; P=.0052; Figure 1).
During normal hematopoiesis, we observed that RORa expression decreased with the stages of cellular maturation and is highly expressed in cells of AML patients with complex karyotype (BloodSpot, 2018). We noted that RORa mRNA expression varied across patients suggesting differences between AML subtypes. Expression levels were also confirmed to be different across AML subtypes in vitro by using cell line models (K-562, KG-1, OCI-AML3, U-937, THP-1, MOLM-13). In particular, analysis of TCGA data showed higher mean RORa expression in DNMT3A mutant (MT) (626 RPKM) compared to mean RORa expression in CEBPAMT (243 RPKM; P=0.026), NPM1MT (167 RPKM; P=0.012), and FLT3MT (236 RPKM; P=.003) AML patients.
We then analyzed the sensitivity of cell lines to the commercially available synthetic RORa /RORγ ligand, SR1078. Myeloid lineage cells U-937 and KG-1 were used as a cellular model. Cells in logarithmic phase were treated with increased concentrations of the RORa /RORγ agonist SR1078 (from 3 nM to 30 mM) for 24 hours. Cell viability was measured by MTT tetrazolium reduction assay. Maximal growth inhibition was reached at 30 µM for U-937 (80%) and KG-1 (75%), respectively. We then combined SR1078 with MEC to evaluate whether in vitro SR1078 increased MEC growth inhibition. The addition of SR1078 to MEC significantly decreased cell viability in KG-1 cells compared to MEC alone (82% vs. 19%, P=.029).
Our study suggests that increased RORa expression may be associated with improved survival in patients with R/R AML and that RORa may be a potential therapeutic target in AML.
Gerds:Celgene Corporation: Consultancy, Research Funding; Imago Biosciences: Research Funding; Pfizer: Consultancy; CTI Biopharma: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Roche: Research Funding; Sierra Oncology: Research Funding. Mukherjee:Bristol-Myers Squibb: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Partnership for Health Analytic Research, LLC (PHAR, LLC): Consultancy; McGraw Hill Hematology Oncology Board Review: Other: Editor; Projects in Knowledge: Honoraria. Nazha:Jazz Pharmacutical: Research Funding; Incyte: Speakers Bureau; Novartis: Speakers Bureau; MEI: Other: Data monitoring Committee; Tolero, Karyopharma: Honoraria; Abbvie: Consultancy; Daiichi Sankyo: Consultancy. Maciejewski:Alexion: Consultancy; Novartis: Consultancy. Sekeres:Millenium: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees. Advani:Abbvie: Research Funding; Pfizer: Honoraria, Research Funding; Glycomimetics: Consultancy, Research Funding; Amgen: Research Funding; Macrogenics: Research Funding; Kite Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal