
Introduction:
Molecular MRD monitoring in NPM1 mutant (mut) AML is now standard of care in many countries as prospective clinical trials have demonstrated that suboptimal reduction and/or rising levels of NPM1mut transcript are associated with disease relapse (Ivey, NEJM 2016; Balsat, JCO 2017). Higher levels of NPM1mut MRD positivity are associated with poorer outcomes after allogeneic hematopoietic stem transplantation (allo-HSCT) (Dillon, EHA 2019). Data regarding the effectiveness of pre-emptive MRD intervention is scant. A reduction in NPM1mut MRD was reported in 17/33 (52%) receiving azacitidine (Platzbecker, Lancet Oncol 2018). Among patients with NPM1mut molecular relapse in the NCRI AML17 trial, 16/27 (59%) achieved MRD negativity with 1-2 cycles of FLAG-Ida salvage chemotherapy (manuscript in preparation). As the BCL-2 inhibitor venetoclax in combination with low-dose cytarabine (LDAC) or hypomethylating agents (HMA) are known to be highly active in NPM1mut AML (Wei, JCO 2019; Strickland, EHA 2018), we hypothesized that venetoclax-based regimens could represent an effective low intensity option for patients with persistent or rising NPM1mut MRD.
Methods:
Consecutive patients with NPM1mut AML from 4 sites treated with venetoclax-LDAC/HMA for MRD eradication were included if previously received at least 1 cycle of intensive chemotherapy and achieved complete remission. cDNA was analyzed on bone marrow aspirate ± peripheral blood samples using a NPM1 mutant specific RT-qPCR (Ivey, NEJM 2016) according to Europe Against Cancer guidelines (Gabert, Leukemia 2003). Results are expressed as MRD level relative to the diagnostic sample (%) with both samples normalized to a control gene (ABL).
Results:
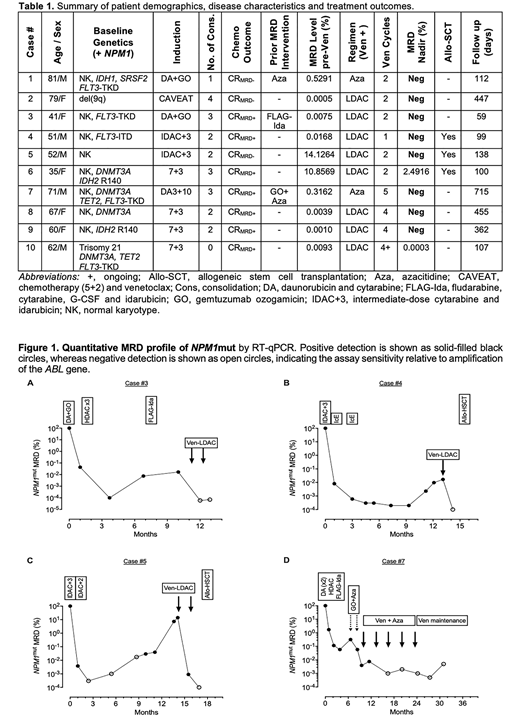
A total of 10 patients were treated with venetoclax (600 mg PO daily D1-14) in combination with LDAC (20 mg/m2 SC twice daily D1-10; n=8), or venetoclax (400 mg PO daily D1-14) azacitidine (75 mg/m2 SC daily D1-7; n=2) as summarized in Table 1. If concurrent posaconazole was used, the dose of venetoclax was adjusted to 100 mg PO daily. The median age of the cohort was 61.2 years (range 31-81). All patients had intermediate risk cytogenetics, including 8 with normal karyotype at diagnosis. Concurrent mutations in addition to NPM1 are shown in Table 1. The number of consolidation cycles received prior to venetoclax ranged from 0-4 and typically included high-dose cytarabine. Three patients had failed other MRD-directed therapies prior to treatment with venetoclax.
Six patients (Cases #1-6) received venetoclax-LDAC/HMA for NPM1mut molecular relapse after complete molecular remission (CRMRD-), or progression after molecular persistence at low copy number (CRMRD+). Five of six (83%) achieved CRMRD- after only 1-2 cycles, with ongoing CRMRD- after a median of 134 days (range 59-447). One patient had failed to respond to FLAG-Ida but achieved CRMRD- after just 1 cycle of venetoclax-LDAC (Figure 1A). Three patients proceeded to subsequent allo-HSCT, including 2 in CRMRD- (Figures 1B and C). Follow up post-HSCT is ongoing (<60 days).
Four patients (Cases #7-10) were treated for molecular persistence after completion of induction and 0-3 cycles of consolidation chemotherapy. All 4 patients responded, including 3 (75%) who achieved CRMRD-. Case #7 (Figure 1D) had received prior gemtuzumab ozogamicin and azacitidine without molecular response but then achieved CRMRD- after receiving venetoclax-azacitidine, which was sustained for >23 months without an allo-HSCT.
Venetoclax in combination with LDAC/HMA was well tolerated. Febrile neutropenia occurred in 2 patients (20%) and one patient had grade 4 sepsis. Grade 4 neutropenia occurred in 70% (median duration 10 days) and grade 4 thrombocytopenia in 40% (median duration 16 days).
Conclusion:
In the setting of molecular NPM1mut MRD relapse or persistence, venetoclax in combination with LDAC/HMA was highly effective, with MRD reductions in 100% and complete molecular remission in 80%. Future clinical exploration is warranted to validate the role of venetoclax as a pre-emptive approach to suppress persistent or rising NPM1mut MRD.
Dillon:Pfizer: Consultancy, Honoraria; TEVA: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Ivey:Novartis: Honoraria. Vassili:Amgen: Honoraria. Taussig:Celgene: Research Funding. Russell:Jazz: Consultancy, Honoraria, Speakers Bureau; Astellas: Consultancy, Honoraria, Speakers Bureau; DSI: Consultancy, Honoraria, Speakers Bureau; Pfizer Inc: Consultancy, Honoraria, Speakers Bureau. Raj:Jazz: Honoraria, Speakers Bureau; Daiichi Sankyo: Honoraria; Pfizer: Honoraria; MSD: Honoraria; Celgene: Honoraria; Novartis: Honoraria, Speakers Bureau; Mallinckrodt: Honoraria, Speakers Bureau. Fong:Pfizer: Consultancy, Speakers Bureau; Novartis: Speakers Bureau; Amgen: Consultancy, Research Funding, Speakers Bureau; Astellas: Consultancy. Grigg:Janssen: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees; Roche: Other: Travel. Knapper:Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Honoraria; Jazz: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Tolero: Membership on an entity's Board of Directors or advisory committees. Wei:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: AHW is a former employee of the Walter and Eliza Hall Institute and receives a fraction of its royalty stream related to venetoclax, Research Funding, Speakers Bureau; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Macrogenics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Honoraria, Research Funding; Janssen: Honoraria.
Venetoclax is a BCL-2 inhibitor that is FDA-approved in some indications. This presentation will focus on venetoclax as a MRD-directed therapy in AML, which is not an approved indication.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal