Background: Blinatumomab is approved in Europe for adult and pediatric patients (pts) with relapsed and/or refractory Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukemia (R/R Ph- BCP-ALL), and for adult pts with minimal residual disease (MRD)-positive Ph- BCP-ALL. Prior to country-specific reimbursement, blinatumomab was made available to pts who met pre-specified criteria via an expanded access program: this included both adult and pediatric pts with a diagnosis of R/R Ph- BCP-ALL, R/R Ph+ BCP-ALL, or MRD-positive Ph-/Ph+ ALL. Here, we describe adults with R/R Ph- BCP-ALL enrolled in this retrospective observational study (NEUF) in specific European countries, with reference to their characteristics, blinatumomab usage and effectiveness.
Methods: Eligible pts initiated blinatumomab in the expanded access setting between 1 Jan 2014 and 31 Dec 2016. Data were extracted from medical notes. Pts were followed from blinatumomab initiation until death, entry into a clinical trial, end of follow-up, or the end of the study period (30 June 2017), whichever occurred first. Adverse events were reported separately, according to local regulations. Calculation of percentages excluded patients with missing data, unless otherwise indicated.
Results: In total, 253 adult pts were enrolled (113 in Italy, 45 in Russia, 53 in Spain, 33 in France, and 9 in the UK): prior to blinatumomab initiation 106 (43%) had a diagnosis of R/R Ph- BCP-ALL, 32 (13%) had R/R Ph+ BCP-ALL, 109 (44%) had MRD positive ALL (either Ph- or Ph+), and 6 (2%) had diagnosis data missing.
Among R/R Ph- BCP-ALL pts (n=106), 47% were female and median age was 36.5 years (interquartile range [IQR]: 24.0, 52.0). Forty-one percent (n=43) had prior allogeneic hematopoietic stem cell transplant ((HSCT). The median number of prior salvage therapies was 1.0 (range: 0.0, 2.0). At blinatumomab initiation, 64 (60%) experienced a relapse and 42 (40%) were refractory. At least half (53%, n=54) of pts were treated with pre-phase and 89% (n=93) with pre-medication with dexamethasone. Within two cycles of blinatumomab, 54 (51%) pts achieved complete remission (CR) with full/partial/incomplete recovery of peripheral blood counts. Among patients achieving CR and who had evaluable MRD (n=33), 85% (n=28) had MRD response (16 with non-detectable MRD and 12 with MRD <10-4). Following blinatumomab, 41% (n=43) pts proceeded to HSCT, among whom 77% (n=33) achieved CR prior to transplant. Median time from CR to HSCT was 4.6 months (range: 0.2, 7.4).
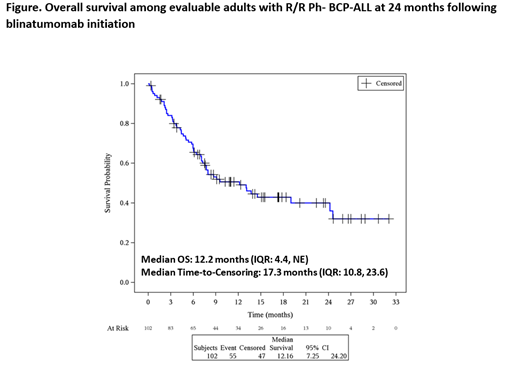
Median relapse-free survival in R/R Ph- BCP-ALL was 11.0 months (range: 0.0, 15.4). Among the 22 adults who experienced blinatumomab relapse and were also tested for CD19 expression and 95% (n=21) were positive. At 24 months following blinatumomab initiation, the Kaplan-Meier (KM) median estimate of overall survival (OS) among 102 evaluable pts was 40% (95% confidence interval [CI]: 29, 51) (Figure); when censoring for HSCT, the OS probability (KM median estimate) was 36% (95% CI: 19, 53): median follow-up time in these analyses was 17.3 months (IQR: 10.8, 23.6) and 9.5 months (IQR: 4.4, 24.2), respectively. The 3-month non-relapse mortality following HSCT post-blinatumomab was 11% (95% CI: 4, 29).
Conclusions: This is the largest documented cohort of R/R Ph- BCP-ALL patients treated with blinatumomab in real-world clinical practice. A high proportion of pts achieved CR, and over one-third could proceed to HSCT. Over one-third of pts were still alive 24 months after blinatumomab initiation. The results are widely consistent with published results from clinical trials and they confirm the effectiveness of blinatumomab in this real-world setting.
Boissel:NOVARTIS: Consultancy. Chiaretti:Incyte: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees. Rambaldi:Novartis: Membership on an entity's Board of Directors or advisory committees, Other: travel support, Speakers Bureau; Jazz: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: travel support, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: travel support, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Omeros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Italfarmaco: Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding, Speakers Bureau. Bassan:Pfizer: Honoraria; Incyte: Honoraria; Amgen Inc.: Honoraria; Shire: Honoraria. Papayannidis:Amgen: Honoraria; Incyte: Honoraria; Shire: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Teva: Honoraria. Alam:Amgen: Employment, Equity Ownership. Brescianini:Amgen: Employment, Equity Ownership. Pezzani:Amgen: Employment, Equity Ownership. Kreuzbauer:Amgen: Employment, Equity Ownership. Foà:Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Shire: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Shire: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal