Introduction:
Activating internal tandem duplication of FMS-like tyrosine kinase 3 (FLT3-ITD) is one of the most frequently mutations in acute myeloid leukemia (AML) patients with a frequency of 20-30%. Although AML with FLT3-ITD mutation is not recognized as a distinct entity in the World Health Organization classification system, AML patients with FLT3-ITD usually have high white blood cell count, high risk in poor prognostic outcome and are benefited from FLT3 inhibitors. However, the outcome prediction of AML with FLT3-ITD is still not very clear in patients undergoing allogeneic hematopoietic cell transplantation (alloHCT). Recent studies showed that the depth of remission, mostly accessed by flow cytometry (FCM) based measurable residual disease (MRD) and next generation sequencing based molecular MRD, was highly predictive for relapse in AML before alloHCT. The depth of remission is considered an important criterion for evaluating AML relapse before alloHCT. Hence, FLT3-ITD remission depth showed a potential application in evaluating FLT3-ITD subtype's prognosis. Therefore, in this retrospective study we tried to evaluate prognostic impact of FLT3-ITD remission depth in AML before alloHCT.
Methods:
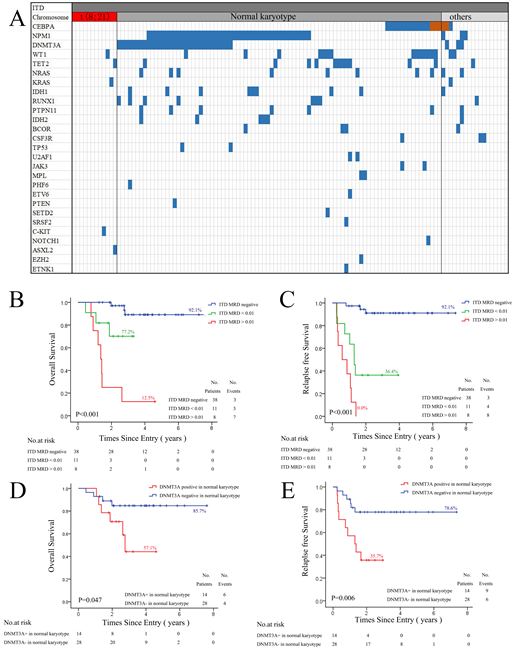
A total of 117 FLT3-ITD positive patients were obtained in our study if they were 18 years or older, had a diagnosis of AML excluding acute promyelocytic leukemia, received standard induction chemotherapy comprising anthracycline and cytoarabine, at first affiliated hospital of Soochow University between September 2011 to January 2018 . All patients were examined a panel of 51 genes mutation by next generation sequencing and FLT3-ITD allelic ratio (AR) in newly diagnosed bone marrow samples (Fig1 A). 57 patients received two courses of standard chemotherapy and immediate alloHSCT in four months. The median time from first chemotherapy to alloHSCT was 99 days. FLT3-ITD was also examined direct after secondary chemotherapy with the sensitivity level of 0.005. Distribution of OS and RFS were estimated by Kaplan-Meier method. Difference of FLT3-ITD positive ratio in FLT3-ITD subtypes was analyzed by Chi-squared test. The two-sided level of significance was set at p<0.05. The deadline for follow-up was May 31, 2019, with a median follow-up time of 24 (5-83) months.
Results:
In our cohort, the median FLT3-ITD AR of 117 patients was 0.53 (range 0.02-7.72) in newly diagnosis. Interestingly, patients with DNMT3A mutation (0.615, range 0.04-7.72) had a higher FLT3-ITD AR than those of patients with CEBPA double mutation (0.19, range 0.02-0.74), with statistical difference (p=0.007). Patients with DNMT3A mutation also had lower complete remission rate than those of patients with NPM1 mutation and DNMT3A wildtype after first chemotherapy (x2=5.987, P=0.018).
In 57 patients undergoing alloHSCT, we found there were no differences in OS and RFS among patients classified by FLT3-ITD AR or concomitant NPM1 mutation. However, patients with FLT3-ITD positive had a poorer OS (p<0.001) and RFS (p<0.001) than those of patients with FLT3-ITD negative accoding to the FLT3-ITD states after secondary chemotherapy. In addition, patients with FLT3-ITD AR>0.01 had a poorer OS and EFS than those of patients with FLT3-ITD AR<0.01, indicating FLT3-ITD remission depth is helpful in prognosis prediction for patients undergoing alloHCT (Fig1 B,C). Moreover, we found patients with DNMT3A mutation had a higher FLT3-ITD positive ratio than those of patients with NPM1 mutation after secondary chemotherapy. This result was also found in patients with normal chromosome karyotype (NK). There was no FLT3-ITD positive in patients with CEBPA double mutation or t(8;21) after secondary chemotherapy in our cohort, while 4 of 8 patients with chromosome karyotype abnormalities (no favorable) had FLT3-ITD positive after secondary chemotherapy. From these results above, we finally found patients with DNMT3A mutation had a poorer OS and EFS in NK patients undergoing alloHCT(Fig1 D,E).
In conclusion, our results suggested FLT3-ITD remission depth is useful in prognosis prediction for AML patients with FLT3-ITD before alloHCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal