Introduction
AML therapy requires intensive and highly immunosuppressive cytotoxic therapy leading to prolonged neutropenia. Prolonged neutropenia leads to highly invasive bacterial and fungal infections causing high rates of infection related morbidity and mortality. Even with improved antimicrobial and antifungal therapy, infectious complications remain a common cause of treatment related morbidity and mortality. There is a large need for strategies to reduce the toxicity of AML therapy and infectious complications in particular. We proposed that post induction infusion of non HLA-matched, off-the-shelf (OTS) ex-vivo expanded cord blood progenitor cells may provide rapid, short term bridging hematopoiesis and truncated period of neutropenia. Here we describe results of a pilot study assessing the safety of infusing this OTS product following FLAG chemotherapy in pediatric patients.
Methods
Between March 2013 and July 2016, 7 pediatric patients with relapsed/refractory AML or acute leukemia of ambiguous lineage (ALAL) were enrolled in this pilot study to evaluate the use of the OTS product as supportive care following FLAG chemotherapy (n=4 at Fred Hutchinson Cancer Research Center/Seattle Children's Hospital and n=3 at Children's Healthcare of Atlanta). All patients received 1 round of FLAG chemotherapy: total dose of 35 mcg/kg of G-CSF, 150 mg/m2 of Fludarabine, and 10 g/m2 of Cytarabine (Ara-C). OTS products were infused no sooner than 24 hours after last dose of Ara-C. The primary study endpoint was safety of infusing a single OTS product as measured by a. infusion toxicity (> grade 3), b. allo-immune platelet refractoriness, c. treatment related mortality, d. delayed marrow recovery beyond day 42, and e. transfusion related GVHD. Chimerism studies were done on cycle days 15 and 22 (+/- 1 day). To monitor for alloimmunization, pre- and post-treatment panel reactive antibody (PRA) tests were conducted. Bone marrow evaluations were performed at the end of the chemotherapy cycle. Absolute neutrophil count (ANC) of both 100 and 500 were used to assess myeloid recovery. Infectious complications were collected.
Results
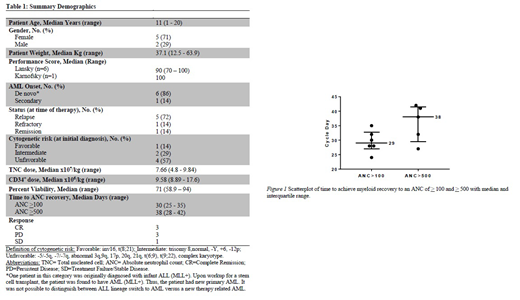
Table 1 shows demographics, disease characteristics and disease status at the time of study enrollment. There were no infusional toxicities reported. No alloimmunization, treatment related mortality, delayed marrow recovery, or transfusion related GVHD occurred in any patient. No adverse events were attributed to the OTS product. Chimerism results showed lack of persistence of OTS products after cycle day 15 (7 days post-infusion of OTS product).
The median TNC dose infused was 7.66x107 (range 4.8-9.84 x107), and the median CD34+ cell dose was 9.58x106 (range 8.89-17.6 x106) (Table I). Six of the 7 patients demonstrated an ANC > 100. One patient relapsed prior to count recovery with 33% blasts in the marrow. The median time to reach an ANC > 100 was cycle day 30 (range 25-35). Five patients recovered an ANC > 500. The patient who did not reach this point relapsed on cycle day 33 with 80% blasts in the marrow. For these 5 patients, the median time to reach an ANC > 500 was cycle day 38 (range 28-42) (Fig 1).
Of the 7 patients enrolled on the study, 3 achieved complete remission (CR), 3 had persistent disease after initial response, and 1 had treatment failure. Infectious complications occurred in 4 patients and included 3 bacteremias in 3 patients (Strep viridans, E. coli, and Staph aureus), one RSV URI (in patient with concurrent Strep viridans bacteremia) and a single case of C. difficile colitis. There were no induction deaths with all patients completing induction therapy and all but one receiving further treatment. Overall, all study patients met pre-specified safety criteria.
Conclusion
These preliminary data demonstrate safety of the use of OTS products in the setting of intensive chemotherapy in pediatric patients with relapsed/refractory high-risk AML. More comprehensive randomized studies will enable evaluation of efficacy of OTS products in decreasing infection related morbidity and mortality. Given demonstrated safety of this product, its use in newly diagnosed AML should be considered to eliminate the substantial infection related toxicities.
Qayed:Bristol-Myers Squibb: Honoraria. Milano:ExCellThera: Research Funding; Amgen: Research Funding. Delaney:Nohla Therapeutics: Employment, Equity Ownership; Biolife Solutions: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal