Background:
Lintuzumab Ac225 is a radiolabeled anti-CD33 antibody with demonstrated single-agent activity in AML. The drug is composed of an alpha emitting isotope, Ac225, conjugated with the humanized anti-CD33 monoclonal antibody lintuzumab. In a prior study, lintuzumab Ac225 monotherapy was safely administered to elderly/unfit AML patients with toxicities primarily limited to prolonged myelosuppression. Nearly 70% of patients achieved a CR/CRi at the highest dose level (2uCi/kg). We hypothesized that lintuzumab Ac225, when added to the salvage chemotherapy regimen CLAG-M (cladribine, cytarabine, G-CSF, and mitoxantrone), would be tolerable and would improve remission rates in the treatment of relapsed/refractory AML (RR-AML). CLAG-M salvage was selected based on favorable institutional outcomes (CR/CRi: 54%, Mushtaq et al, ASH 2018). This novel investigator-initiated phase I study is the first study to combine radioimmunotherapy and intensive chemotherapy in patients with RR-AML.
Patients and Methods:
Eligible patients include medically fit, RR-AML patients aged 18 years and older, with adequate organ function. In addition, more than 25% of leukemic blasts must have been CD33 positive by flow cytometry. Induction consisted of G-CSF, 300mcg/d, given D1-6, cladribine 5mg/m2, given D2-6, cytarabine 2g/m2, given D2-6, and mitoxantrone 10mg/m2, given D2-4. Lintuzumab Ac225 was administered as a single dose on either days 7, 8, or 9. Cohort 1 received lintuzumab Ac225 at a dose of 0.25uCi/kg, and cohort 2 received a dose of 0.50uCi/kg. Only one induction course was administered, and subsequent treatments were up to physician discretion.
Results:
Nine patients with a median age of 59 years (range 47-73 yrs) have been enrolled. Patients had a median of 2 (range 1-4) prior, anti-leukemic treatments, including 4 patients who relapsed following allogeneic HCT. Four patients (44%) had intermediate risk cytogenetics, and five patients (56%) had adverse risk features. Median blast CD33 expression was 73% (range 32-100%).
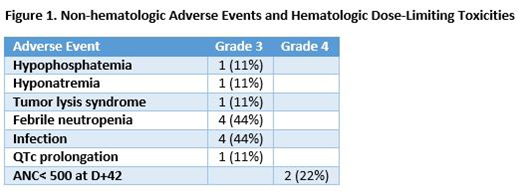
All patients completed one cycle of lintuzumab Ac225 with CLAG-M. Three patients enrolled into cohort 1 (0.25mcg/kg), and six patients enrolled into cohort 2 (0.5mcg/kg). Among all enrolled patients, Grade 3 or greater AEs include febrile neutropenia (n=4), infection (n=4), QTc prolongation (n=1), hypophosphatemia (n=1), hyponatremia (n=1), and tumor lysis syndrome (n=1). In two responding patients enrolled to cohort 2, one patient had prolonged time to neutrophil recovery (ANC>500 at 53 days), and a second patient, who was post-HCT, had an ANC recovery to 300 by day 42, and then received donor CD34+ stem cells to aide in count recovery. Figure 1 summarizes toxicities. All responding patients with a platelet count>50k prior to therapy, had a post-treatment platelet count >50k within 42 days. No mortalities were observed on study.
Among cohort 1 patients (n=3), one patient achieved CR, one patient had a >50% blast reduction to 6.2% blasts with neutrophil recovery and platelet improvement to 70k, and the third patient had no response. Overall, 33% (1/3) achieved a CR. Among patients enrolled into cohort 2, two patients achieved CR, two patients achieved CRp, one patient achieved CRi, and one patient had no response. Overall, 83% (5/6), achieved CR/CRp/CRi. After, three patients on study subsequently went onto allogeneic HCT, two patients initiated donor lymphocyte infusions (DLI), and one patient underwent CD34+ stem cell boost.
Conclusion:
Combining lower doses of lintuzumab Ac225 with salvage CLAG-M chemotherapy appears to have a clinically acceptable safety profile. With dose escalation, increased myelosuppression has been noted alongside, but also highly encouraging efficacy results for RR-AML. As two patients in Cohort 2 met DLT criteria due to prolonged ANC recovery, this study will be amended to mandate the addition of G-CSF two weeks after Lintuzumab Ac225 treatment. Tentatively, this study will plan to extend the ANC recovery observation period to 60 days. Depending on safety and further efficacy data, this may lead to further dose escalation, or potentially an expanded number of patients in a Phase 2 study.
Abedin:Actinium Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Honoraria; Agios: Honoraria; Helsinn Healthcare: Research Funding; Pfizer Inc: Research Funding. Runaas:Agios: Honoraria; Blueprint Medicine: Honoraria. Michaelis:Incyte: Consultancy, Research Funding; Novartis: Consultancy; BMS: Research Funding; TG Therapeutics: Consultancy, Research Funding; JAZZ: Other: Data Safety Monitoring Board, uncompensated, Research Funding; Macrogeneics: Research Funding; Millenium: Research Funding; ASTEX: Research Funding; Pfizer: Equity Ownership, Research Funding; Celgene: Consultancy, Research Funding; Bioline: Research Funding; Janssen: Research Funding. Atallah:Helsinn: Consultancy; Jazz: Consultancy; Takeda: Consultancy, Research Funding; Pfizer: Consultancy; Jazz: Consultancy; Helsinn: Consultancy; Novartis: Consultancy. Hamadani:Celgene: Consultancy; Merck: Research Funding; Sanofi Genzyme: Research Funding, Speakers Bureau; Otsuka: Research Funding; Janssen: Consultancy; Medimmune: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Takeda: Research Funding; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal