Background: Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) is a rare subtype of acute lymphoblastic leukemia with a poor long-term prognosis. Recent studies suggested that the addition of tyrosine kinase inhibitor (TKI) to the traditional chemotherapy treatment has significantly improved Ph+ ALL patients response rates, disease-free survival, and overall survival (OS). Dasatinib, a second-generation tyrosine kinase inhibitor, can pass the blood-brain barrier and possesses a stronger inhibitory effect on both SRC kinase and BCR-ABL. In theory, it might be beneficial in Ph+ ALL treatment. This prospective, single-arm study assesses the efficacy of a combination of dasatinib and pediatric-inspired regimens in Ph+ ALL patients.
Methods: 30 patients from the Institute of Hematology and Blood Diseases Hospital were enrolled in this study from January 2016 to April 2018. Eligible subjects were newly diagnosed Ph+ ALL adult patients. Chemotherapy regimens were initiated after the pediatric-inspired regimens, and standard induction chemotherapy was given for four weeks. Seven courses of consolidation or hematopoietic cell transplantation (HCT) were given to those who have achieved hematological complete remission (HCR). The primary objectives of this study were the HCR and molecular complete response (MCR), the major molecular response (MMR), the overall survival (OS), and the hematologic relapse-free survival (HRFS). The median follow-up time was 28 months. The trial registration number is NCT02523976.
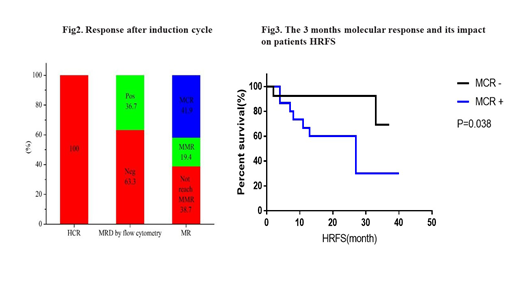
Results: 30 subjects were enrolled in this study with a median age of 37.5 years (range 19-50 years). All patients achieved HCR after four weeks of induction therapy with a cumulative MCR rate of 87.5%(21/24). The median HRFS and median OS were 19.5 (range 2-40 months) and 21 (range 7-41 months), respectively. The molecular response, assessed through monitoring of BCR-ABL transcript expression, revealed that 61.3% of the patients reached MMR and MCR after three months of treatment. Additionally, the results indicated that patients who achieved MCR in the first three months had a better HRFS (p=0.038). Fifteen of the patients (50%) proceeded to stem cell transplantation (SCT) within the first HCR period (SCT in HCR1). Only 13.3% (2/15) of the SCT cohort relapsed, and 20% (3/15) died. It is worth mentioning that the SCT in HCR1 cohort had better HRFS (P=0.03). Most adverse events were reversible, and none of the subjects had pulmonary hypertension.
Conclusion: These findings indicate that an early MCR (3 months) has a positive impact on patients survival and that Dasatinib, combined with pediatric-inspired regimens, is effective and leads to a high MCR in patients with newly diagnosed Ph+ALL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal