Introduction
As survival rates for acute lymphoblastic leukaemia (ALL) in children and young people improve, the need to understand and mitigate toxicity becomes increasingly important. The contribution of toxicity to mortality or significant morbidity is well described. However, the impact of dose reductions, drug omissions or delays in treatment which result from toxicity is poorly understood, other than the established association between effective count suppression during maintenance chemotherapy and a reduction in relapse rate. We report the significance of treatment delays and dose reductions in induction chemotherapy in the UKALL2003 trial.
Methods
UKALL2003 was a prospective multicenter phase 3 study recruiting patients aged 1-24 years with newly diagnosed Philadelphia chromosome negative ALL in the UK. Data regarding 4 different protocol deviations were prospectively collected at the end of each block of therapy; a) not given allocated regimen, b) <90% of one or more of the protocol drugs administered, c) change of Asparaginase product and d) delay of > 1 week in commencing the next treatment block. Univariable (UVA) and Multivariable (MVA) Cox regression was used to assess the association of each deviation with risk of relapse. To control for immortality bias, this was assessed at the end of each therapy block with only patients alive and relapse free at the end of the block included in the analysis. Patients with Down Syndrome were excluded from all analyses.
Results
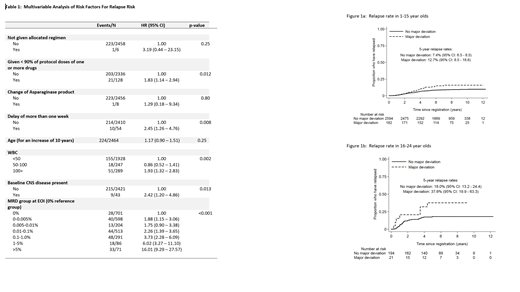
Of 2991 patients without Down Syndrome completing induction chemotherapy, 9 patients did not receive the allocated regimen, 1558 patients received <90% of one or more drugs, 9 patients were switched to an alternative Asparaginase product and 72 had a greater than 1 week delay in commencing consolidation. On UVA, reduction of >90% of one or more drugs and delay of greater than 1 week was significantly associated with an increased risk of relapse with hazard ratios of 1.70 (95% CI 1.11 - 2.60, p=0.014) and 1.99 (95% CI 1.14 - 3.48, p=0.013) respectively. On MVA (complete cases, n=2464), including other factors of established prognostic significance, both deviations remained significantly associated with an increased risk of relapse (Table 1). The impact of a major deviation (defined here as dose reduction of >90% of one or more drugs or treatment delay of >1week) was seen in both the paediatric (<16 years) and older (16-24 years) groups with a slightly larger effect in the older group (HRs 1.73 and 2.40 respectively), though the interaction between age and deviation was not significant (p=0.49)( Figure 1). Patients with dose omissions >90% of one or more drugs in induction were also more likely to have major deviations in subsequent blocks of therapy (p<0.001) and remained at significantly higher risk of relapse when starting the maintenance phase of treatment (n=2343, HR: 1.81 (1.06 - 3.09, p = 0.03).
Conclusion
Major deviations in the delivery of induction chemotherapy on UKALL2003 were significantly associated with a greater risk of further deviations in subsequent blocks of therapy and a higher risk of relapse. Further improvement in efficacy of ALL therapy in children and young people will require a greater understanding of which toxicities lead to major deviations in therapy and strategies developed to mitigate these risks.
Rowntree:Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal