Introduction
Ibrutinib, a first-in-class oral covalent inhibitor of Bruton's tyrosine kinase (BTK), has been FDA-approved for the treatment of mantle cell lymphoma, chronic lymphocytic leukemia (CLL) and Waldenström's macroglobulinemia. In CLL, 80% overall response rate (OR) as a monotherapy or 96% OR in the combination with BCL-2 selective inhibitor venetoclax demonstrates a promising regimen for CLL patients. However, ibrutinib only yields to a modest (38%) OR in follicular lymphoma (FL) trial, especially doing poorly in patients with NF-kB constitutive activation via CARD11 mutation. To better understand ibrutinib resistance mechanism, we established a resistance model using sensitive follicular lymphoma cell line DOHH2, and characterized the gene expression profile and signaling pathway alteration, and tested potential approach to overcome the resistance.
Methods
1. DOHH2-IR (ibrutinib resistant) cells were established by exposing to the increasing concentrations of the drug over 3 months and confirmed by cell viability assays.
2. RNA-seq was performed to reveal the transcriptome alteration between DOHH2 and DOHH2-IR cells.
3. Cell sensitivity to BCL-2/BCL-xL inhibitors was assessed by CTG assays.
Results
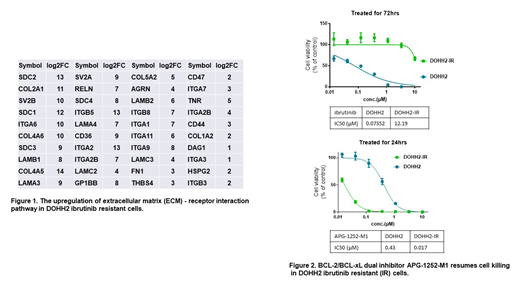
Parental DOHH2 and resistant DOHH2-IR cells were first confirmed ibrutinib sensitivity/resistance by CTG assay, which showed an IC50 of 0.074 uM for DOHH2, but 160 fold greater for DOHH2-IR at 12.19 uM. To decipher the mechanisms underlying the induced ibrutinib resistance, whole-transcriptome RNA-seq was performed on parental and resistant cells. Unsupervised hierarchical clustering showed significant alterations in gene expression signatures. Among them, 4,925 genes were significantly up-regulated and 3,727 genes were down-regulated. Kyoto Encyclopedia of Genes and Genome (KEGG) analysis showed that the components of BCR signaling pathway had been significantly downregulated in the resistant cells, so were those in pro-survival NF-kB and mTOR pathways, anti-apoptotic BCL-2 and BFL-1 genes, and TP53 pathway; in contrast, BCL-xL, MAP kinase and extracellular matrix (ECM)-receptor interaction genes, especially those integrin family genes, were increased (Figure 1).
The upregulation of BCL-xL gene expression prompted us to investigate if inhibiting BCL-xL would resume cell sensitivity to killing. We thus treated cells with APG-1252-M1 (ester hydrolyzed active metabolite of APG-1252 for in vitro study), a novel BCL-2/BCL-xL dual inhibitor developed by Ascentage. In comparison, we also treated cells with BCL-2 selective inhibitor APG-2575, which is also a proprietary compound by Ascentage, and its reference compound ABT-199. APG-2575 and ABT-199 partially restored cell death in the resistant cells, with IC50s of 0.65 uM and 1.19 uM, respectively, which were greater than parental cells with IC50s of approximate 0.05 uM. However, the dual inhibitor APG-1252-M1 effectively killed DOHH2-IR cells with an IC50 of 0.017 uM, and cell death was potently elicited within 24 hours (Figure 2). This finding is consistent with RNA-seq data, that BCL-2 is downregulated, while BCL-xL is induced during ibrutinib resistant selection.
Conclusions
In an ibrutinib-resistant DOHH2 model, we show that reprogram of BTK and apoptosis pathway is associated with ibrutinib resistance. Instead of mutating BTK or its downstream PLCg2 gene in CLL, resistant cells have selected to bypass the pathway; and unexpectedly, NF-kB pathway is also downregulated in DOHH2-IR, which is contrast to previously report that it is one of resistant mechanisms for ibrutinib.
Evading apoptosis is one of the key mechanisms in which cancer cells can survive or become resistant to therapies. Integrin family proteins or ECM - receptor interactions also play important roles in drug resistance. Further studies are needed to understand how the components of integrin pathway cross-talk with BCL-2 family proteins. However, the potent killing activity conferred by APG-1252 confirms that BCL-xL upregulation is key in this FL ibrutinib resistance model. Collectively, the results suggest that the combination of ibrutinib and BCL-2/BCL-xL dual inhibitor may produce deeper and longer duration response in follicular lymphoma patients.
Deng:Ascentage Pharma Group: Employment. Mao:Ascentage Pharma Group: Employment. Huang:Ascentage Pharma Group: Employment. Rui:Ascentage Pharma Group: Employment. Wang:Ascentage Pharma Group: Employment. Fang:Ascentage Pharma Group: Employment. Yang:Ascentage Pharma Group: Employment. Zhai:Ascentage Pharma Group: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal