Background: In the Fanconi anemia (FA) DNA repair pathway, DNA damage induces the mono-ubiquitination of the FANCI-FANCD2 (ID2) heterodimer by the FA core complex through its inherent E3 ligase activity. The timely deubiquitination of ID2 by USP1-UAF1 deubiquitinase complex is also critically important for the FA DNA repair. UAF1 has a DNA binding activity, which is required for FANCD2 deubiquitination. UAF1 also enhances RAD51-mediated homologous DNA pairing in a manner that is dependent on complex formation with RAD51AP1. UAF1 deficient cells are impaired for DNA repair by homologous recombination (HR).The biochemical and cellular functions of UAF1 DNA binding activity in HR remain elusive.
Methods:UAF1 wild type and DNA binding mutant proteins were purified and used to define its biochemical properties in HR. In vitroD-loop formation and synaptic complex assembly assay were performed to discover the DNA binding of UAF1 in RAD51 recombinase enhancement. U2OS-DR-GFP cell lines with impaired UAF1 or RAD51AP1DNA binding were generated to examine HR efficiency and DNA damage resistance.
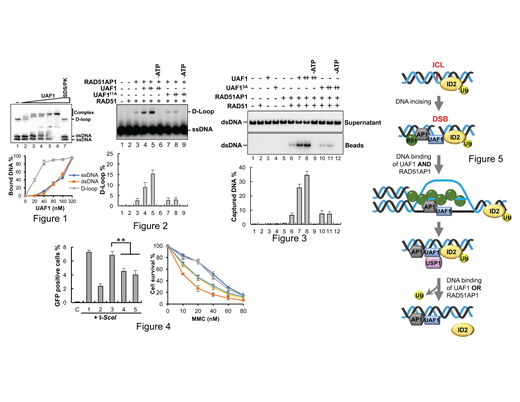
Results:UAF1 preferentially binds an HR-intermediate-like DNA substrate (D-loop, Fig.1). The DNA binding deficient mutant of UAF1 is unable to stimulate RAD51AP1 promotion of RAD51-mediated D-loop (Fig. 2) and the ability to recruit homologous DNA to form the presynaptic complex formation in HR (Fig. 3). In cells, the UAF1 DNA-binding mutant is compromised for the ability to repair DNA damage and to implement HR (Fig. 4). Such activity correlates with the ability to confer resistance to DNA cross linking agents such as mitomycin C (Fig. 4). The DNA binding of UAF1 and RAD51AP1 have a coordinated role in HR-directed DNA damage repair (Fig. 5).
Conclusions: UAF1 DNA binding activity is indispensable for its function in enhancing RAD51-mediated homologous DNA pairing within the context of the UAF1-RAD51AP1 complex. UAF1 DNA binding deficiency causes DNA damage sensitivity and impairs HR efficiency in cells.
Translational Applicability:Our findings reveal a critical role of UAF1 DNA binding in DNA repair and genome maintenance. The identification of UAF1's role in repair will enable targeted efforts to improve molecular approaches for FA therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal