Background: Rivaroxaban is a direct oral anticoagulant (DOAC) with similar efficacy to vitamin K antagonists in the management of non-valvular atrial fibrillation (NVAF) and venous thromboembolism (VTE) but with a more favourable bleeding profile. Recent studies including the landmark COMPASS trial have demonstrated that rivaroxaban possesses cardioprotective and anti-inflammatory properties beyond its well-established anticoagulant effects however, these remain poorly characterized. The effects of FXa inhibition on the generation of circulating extracellular vesicles (EV) are currently unknown. We hypothesize that circulating proinflammatory EV profiles differ in patients treated with rivaroxaban compared to those treated with warfarin and that these changes may represent a novel biomarker for cardioprotective effects mediated by FXa inhibition.
Methods: Patients stably anticoagulated with 20 mg Rivaroxaban (n=15) once daily or warfarin (n=15) (at a target INR of 2.0 - 3.0) who had commenced therapy no sooner than 3 months previously for VTE treatment or NVAF were recruited following informed consent at the Mater Misericordiae University Hospital, Dublin. Demographic and clinical data were collected including patient age, body mass index, smoking and alcohol history, medical comorbidities, white cell and platelet count, creatinine clearance, liver profile, medications, detailed indication for anticoagulation and time since last dose of medication. Patients receiving warfarin had a time in therapeutic range of > 55% and had an INR within target range at the time of sampling.
Exclusion criteria included severe renal impairment (creatinine clearance < 30 ml/min), significant liver impairment, known proinflammatory conditions (including systemic lupus erythematosus, inflammatory bowel disease, rheumatoid arthritis), recurrent VTE, active malignancy, previous stroke or systemic inflammation, antiphospholipid syndrome, strong thrombophilia (eg antithrombin deficiency), individuals aged < 18, patients receiving inhibitory CYP3A4 and P-glycoprotein medications or platelet inhibitors, bleeding or platelet function disorders, and thrombocytopenia (platelet count < 150 x 109/ml)
Total particle counts in platelet poor plasma were measured by Nanoparticle Tracking Analysis (NTA) and flow cytometry. NTA was carried out using a NanoSight NS300 (Malvern) with a camera level of 13 and a detection threshold of 10. 15 x 60 second videos were captured per sample. Flow Cytometry analysis was performed using a CytoFlex LX (Beckman Coulter). Gigamix beads (Biocytex) were used to establish size gates for 100, 300, 500 and 900 nm particles. Samples were analysed in triplicate. Statistical analysis was performed in RStudio (version 1.2.1335) using a two-tailed t-test with a significance level of 5%.
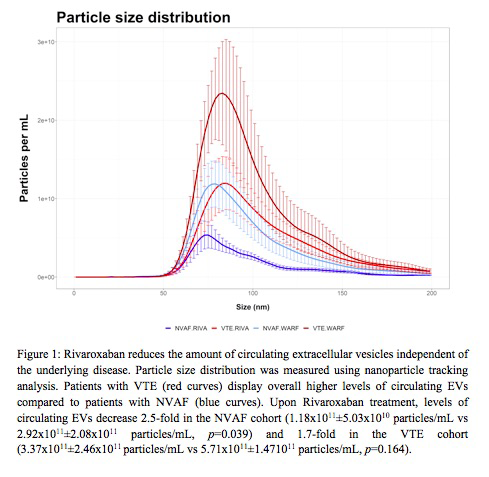
Results and Conclusion: Overall, we observed lower circulating EV levels in patients anticoagulated with FXa inhibitors versus vitamin K antagonists (Figure 1). Within our groups, patients with NVAF exhibited overall lower EV particle counts than VTE patients. Interestingly, EV levels were observed to be further diminished in patients treated with rivaroxaban versus warfarin. In this group, we detected a 2.5-fold decrease in the level of circulating EVs in patients with NVAF (1.18x1011±5.03x1010 particles/mL vs 2.92x1011±2.08x1011 particles/mL, p=0.039) and a 1.7-fold decrease in patients with VTE (3.37x1011±2.46x1011 particles/mL vs 5.71x1011±1.471011 particles/mL, p=0.164), in contrast to warfarin treatment. Collectively, these data suggest that FXa inhibition reduces the generation of circulating EVs, independent of the disease. These findings are of translational relevance towards characterizing cardioprotective mechanisms associated with rivaroxaban therapy.
Ni Ainle:Leo Pharma: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Actelion: Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees. Maguire:Bayer: Research Funding; Leo Pharma: Research Funding; Actelion: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal