Background. In clinical practice, tapering off thrombopoietin receptor agonists (TPO-RA) in immune thrombocytopenia (ITP) is considered if therapy is no longer needed due to a decrease in the disease activity favoring sustained treatment-free responses (TFR). To date, there are no predictors to identify when this approach is likely to be successful, other than earlier start of TPO-RA, and robust platelet responses.
Aim. To evaluate clinical predictors of TFR in a real-world cohort of ITP patients treated with TPO-RA by dealing with confounding variables that could be minimized at the stage of the analysis.
Methods. In this retrospective, multicenter study from 19 secondary and tertiary Spanish hospitals, patients aged >18 years with chronic ITP who had initiated TPO-RA (eltrombopag [EPG] or romiplostim [ROM]) between January 2012 and December 2014 were included. Data were collected from medical records (November 2016 to January 2018) on patient characteristics, history of disease and previous therapies, TPO-RA administration, response and discontinuation.
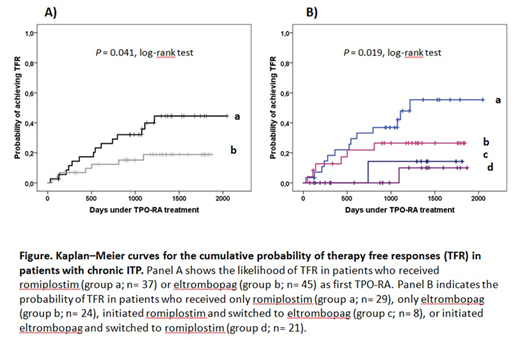
Results. A total of 82 patients with a median age of 63 years (range 19-90 years), 59.9% females initiated TPO-RA (ROM, n=37; EPG, n=45). The median time from diagnosis of ITP to therapy with TPO-RA was 5.5 years (1.1-50.3 years). In all cases the TPO-RA was started with intention to treat indefinitely; the median initial doses of EPG were 350 mg/week (175-525 mg/week), and those of ROM 2.0 μg/kg/week (1.0-7.0 μg/kg/week). The median time on TPO-RA treatment was 2.9 years (0.1 to 5.6 years), and the median follow-up from start of TPO-RA until collection of data was of 3.9 years (1.3 to 5.6 years). A total of 29 patients (35.4%) switched TPO-RA during follow-up. The most frequent cause for switching was lack of efficacy (44.8% of cases -in all cases the initial TPO-RA was EPG-). Due to switching, 58 patients received ROM, and 53 were treated with EPG, yielding 122.3, and 100.8 patient-years of total exposure, respectively. During follow-up almost one half of the patients (47.6%, n=39) stopped TPO-RA. After a median of 1.4 years (0.1-3.3 yrs) under TPO-RA treatment, 19 patients (23.2% of the whole cohort) maintained TFR defined as platelet counts >50x109/l for a median follow-up of 2.8 years (1.5-4.4 years) in the absence of any platelet increasing drug. Remarkably, while the specific TPO-RA that was discontinued did not influence the probability to achieve TFR (P=0.582), there was a trend towards receiving ROM as first TPO-RA and attaining sustained responses (P=0.073), while switching TPO-RA negatively predicted TFR (P=0.010). In univariate analysis with logistic regression, switching TPO-RA was associated with a 6.4 risk of not achieving TFR (P= 0.019). The overall comparison of the Kaplan-Meier curves indicated an association (log-rank P=0.041) among the initial TPO-RA that was prescribed and the probability of TFR (Fig 1A), but the estimated median time to achieve TFR was not reached for either TPO-RA. When switching and initial TPO-RA were considered, patients receiving ROM and not experiencing switching were the best performing group in terms of achieving TFR; the median time to taper off the drug and sustain platelet responses was 3.3 years (95% CI 2.7-4.0 years) (Fig. 1B).
Conclusion. In this observational research analysis, we have tried to minimize the possible bias of some studies that could mistakenly attribute TPO-RA induced TFR, when in fact it may be the natural course of the underlying disease. By analyzing a group of chronic ITP patients with a particularly poor baseline prognosis (median time from diagnosis 5.5 years) we address potential confounding variables by disease severity. Our data show that assuring that long duration under TPO-RA therapy is provided (median of 3.3 years), one half of chronic ITP patients treated with ROM and not undergoing switching can achieve TFR.
Mingot-Castellano:Roche: Consultancy; Bayer: Consultancy; Novartis: Consultancy; Takeda: Consultancy; Sobi: Consultancy; Amgen: Consultancy; Novonordisk: Consultancy; CSL Behring: Consultancy. Jarque:Abbie: Consultancy, Speakers Bureau; Alexion: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Grifols: Consultancy; Gilead: Consultancy, Speakers Bureau; CellTrion: Consultancy; Celgene: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Servier: Speakers Bureau; Roche: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; MSD: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Shire: Consultancy, Speakers Bureau; Shionogi: Consultancy, Speakers Bureau. Campos:Novartis: Speakers Bureau; Amgen: Speakers Bureau. Lopez Fernandez:Amgen: Consultancy, Speakers Bureau. Casado:Amgen: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau. Álvarez Roman:Amgen: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Bayer: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; CSL Behring: Consultancy, Speakers Bureau; Sobi: Consultancy, Speakers Bureau; Takeda: Research Funding; NovoNordisk: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal