Background: Acquired thrombotic thrombocytopenic purpura (aTTP) is an acute, life-threatening thrombotic microangiopathy that requires urgent and specialized treatment. Prior to the introduction of caplacizumab, the treatment for aTTP was based on daily therapeutic plasma exchange (TPE; to replenish functional ADAMTS13 enzyme) plus immunosuppression (mainly corticosteroids and rituximab; to suppress anti-ADAMTS13 autoantibody production). TPE combined with immunosuppressive therapy improved outcomes in patients; however, episodes of aTTP are still associated with an acute mortality of up to 20% as these therapies do not have an immediate effect on the pathologic microvascular thrombosis. The primary results of the randomized, double-blind, placebo-controlled phase 3 HERCULES study showed that, in combination with TPE and corticosteroids, caplacizumab shortened the time to platelet count response and reduced the incidence of a composite outcome of TTP-related death, exacerbation, or major thromboembolic events, by inhibiting vWF-platelet interaction and, thereby, stopping the formation of microthrombi. As additional immunosuppression per local practice was permitted in HERCULES, the present analysis aimed to determine whether there was any difference in the efficacy of caplacizumab according to the initial immunosuppression regimen.
Methods: Data of patients participating in HERCULES were stratified based on the type of first-line immunosuppression regimen (i.e. therapy started up to Day 3 of the treatment period) and analyzed descriptively. The main 2 groups analyzed were those receiving corticosteroids only and those receiving a combined regimen of corticosteroids and rituximab. Differences in dose or dosing frequency were not taken into consideration in this descriptive analysis.
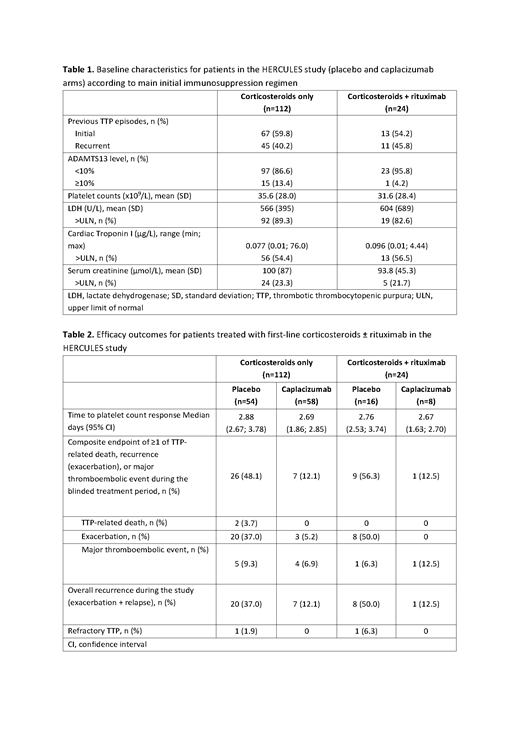
Results: Of the 145 randomized patients in the HERCULES study, 112 (77.2%) patients received only corticosteroids as first-line immunosuppressive therapy, while 24 (16.6%) patients received corticosteroids and rituximab (initiated within the first 3 days of the study). Three patients (2.1%) received another type of initial immunosuppression (cyclophosphamide + corticosteroids [n=1], hydroxychloroquine [n=1], and mycophenolate mofetil + corticosteroids [n=1]), 1 patient (0.7%) started immunosuppression later in the study (cyclophosphamide + corticosteroids), while 5 patients (3.4%) did not receive any immunosuppressive treatment during the study. Baseline characteristics between the main 2 subgroups were well balanced (Table 1). Immunosuppressive therapy intensification occurred in 38 patients (33.9%) initiated on corticosteroids alone (most often addition of rituximab [n=37], others included splenectomy [n=2], bortezomib [n=1], mycophenolate mofetil [n=1]), and in 3 patients (12.5%) initiated on corticosteroids with rituximab (bortezomib [n=1] and mycophenolate mofetil [n=3]). Caplacizumab treatment improved outcomes in patients with aTTP irrespective of the type of initial immunosuppression. Data on time to platelet count response and clinical outcomes are summarized in Table 2. Caplacizumab reduced the rate of the composite endpoint of TTP-related death, exacerbation, and major thromboembolic events during the double-blind treatment period irrespective of baseline immunosuppression regimen. Notably, in the placebo arm, exacerbations occurred in both subgroups to a similar extent, indicating that corticosteroids, with or without rituximab, are not immediately effective. Overall, recurrences (exacerbations or relapses) during the study were also reduced by caplacizumab in both subgroups (Table 2). Two placebo patients died during the treatment period in the corticosteroid only subgroup versus none in the corticosteroid plus rituximab subgroup (one other placebo patient died during the study drug treatment period while receiving another type of immunosuppression).
Conclusion: Immunosuppressive therapy in aTTP aims to control the underlying autoimmune disease, but requires time to take effect; this exposes patients to thrombotic complications and death. Caplacizumab treatment prevents disease exacerbations and death, irrespective of the type of initial immunosuppression used, allowing time for immunosuppressive therapy to take effect.
Pavenski:Ablynx: Honoraria, Research Funding; Shire: Honoraria; Alexion: Honoraria, Research Funding; Octapharma: Research Funding; Bioverativ: Research Funding. Knoebl:Novo-Nordisk: Consultancy, Research Funding; Ablynx/Sanofi: Consultancy; CSL-Behring: Consultancy; Shire/Takeda: Consultancy; Roche: Consultancy. Scully:Shire: Research Funding; Alexion: Consultancy; Ablynx/Sanofi: Consultancy; Shire/Takeda: Consultancy; Novartis: Consultancy. Kremer Hovinga:Siemens: Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology); Shire: Consultancy, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology), Research Funding; CSL-Behring: Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology); Ablynx/Sanofi: Consultancy, Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology); Roche: Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology). Coppo:Shire: Consultancy; Ablynx/Sanofi: Consultancy; Alexion: Consultancy. Peyvandi:Sanofi: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Grifols: Honoraria; Kedrion: Honoraria; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Alnylam: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Octapharma: Research Funding. Cataland:Ablynx/Sanofi: Consultancy, Research Funding; Alexion: Consultancy, Research Funding. Metjian:Genentech: Consultancy, Research Funding; AblynxNV/Sanofi: Consultancy, Research Funding. De La Rubia:Celgene Corporation: Consultancy; AbbVie: Consultancy; Takeda: Consultancy; AMGEN: Consultancy; Janssen: Consultancy. De Winter:Ablynx, a Sanofi company: Employment. de Passos Sousa:Sanofi: Employment. Callewaert:Sanofi (formerly employed by Ablynx, a Sanofi company): Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal