Background
Leg ulcerations are a serious and debilitating complication of sickle cell disease (SCD). Patients with SCD and leg ulcers have biomarkers of severe hemolytic anemia, a state associated with low bioavailability of nitric oxide (NO). Therapies directed at restoring NO bioavailability might prove beneficial. We selected topical sodium nitrite for clinical development due to its safety profile and vascular, anti-microbial, antiplatelet, and pro-keratinocyte functions. The nitrite anion is a vasodilator in vivo by generating NO in tissues with low oxygen tension and pH, conditions that are likely present in chronic wounds. Data from our successful phase 1 study showed improved regional blood flow, decreased ulcer pain, and appeared to improve ulcer healing. The dosing data informed the concentration of active ingredient to be used and suggested sodium nitrite efficacy in SCD patients with leg ulcers (Minniti, CP et al, Lancet Haematol 1, e95-e103).
Study Design and Methods
This is a multicenter, phase 2, prospective, randomized, placebo-controlled study of topical sodium nitrite in patients with SCD and leg ulcers. Primary aim is to determine the safety, tolerability, and effectiveness of twice a week topical application of study ointment for 10 weeks. We hypothesize that sodium nitrite will 1) accelerate wound healing (>25% improvement over placebo arm); and/or 2) decrease pain at the wound site (>20% over placebo). Ulcers measured by ImageJ planimetry software to increase accuracy. The secondary aims are to: a) evaluate the effect of hydroxyurea (HU) on leg ulcer healing in combination with topical sodium nitrite or placebo; b) assess changes in ulcer microbiome after application of sodium nitrite or placebo and how these changes may relate to healing; c) evaluate the dermal composition and microvascular structure in the ulcer beds.
We plan to enroll 50 adults with all SCD genotypes and leg ulcers not individually >100 cm2, such that, after an expected 20% dropout, 40 subjects will complete > 8 weeks of treatment. Exclusion criteria: use of PDE5 inhibitors, NO, L-arginine, nitroprusside, nitroglycerine; acute bacterial infection; pre-existing methemoglobinemia (>3.5% on two different occasions); G6PD deficiency.
Randomization is stratified by HU use to minimize potential confounding effects on study outcomes. Funded by the Food and Drug Administration (FDA) Division of Orphan Drug Development (# FD-R-0005729); active at two sites (ClinicalTrials.gov NCT02863068).
Results
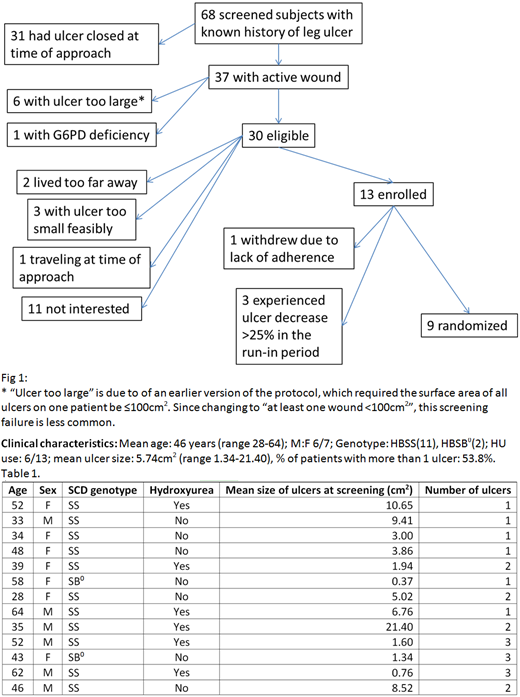
We have screened 68 subjects with known history of leg ulcer: 46% were not eligible as the ulcer was closed at the time of screening. Of the 30 eligible, 13 subjects enrolled (12 at Montefiore Medical Center, 1 at the University of Pittsburgh), with 9 subjects randomized. Reasons for screen failure, other than ulcer closed, are depicted in fig 1. Three subjects experienced ulcer decrease >25% in the run-in period and did not have ointment application as per protocol. One subject withdrew due to lack of adherence. Therefore the overall dropout rate at this time is 33%, higher than the 20% anticipated. No study ointment SAEs have been noted, AEs have been minor and non-ointment related. Most SAEs have been VOCs, expected in this patient population and wound infections.
Discussion
As expected, enrollment of subjects with a rare complication of a rare disease is challenging. The recurrent pattern of ulcers in SCD was the reason why the majority of patients were not able to enroll, as the ulcer was closed at the time of screening. We monitor them closely for possible re-opening. Simplification of protocol-related procedures, such as decrease in number of required visits from twice to once a week by packaging dose-specific blister packs that the patient takes home for self-administration of ointment, has facilitated enrollment. Travel to the center is being addressed. A close relationship between the subjects and study team is essential. Co-location of the wound specialist in the sickle cell clinic and training the research nurse for wound care has helped with recruitment.
Ogu:Vertex Pharmaceuticals: Consultancy. Kato:Novartis, Global Blood Therapeutics: Consultancy, Research Funding; Bayer: Research Funding. Minniti:Doris Duke Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal